Tale of a multifaceted co-activator, hADA3: from embryogenesis to cancer and beyond
- PMID: 27605378
- PMCID: PMC5043578
- DOI: 10.1098/rsob.160153
Tale of a multifaceted co-activator, hADA3: from embryogenesis to cancer and beyond
Abstract
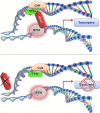
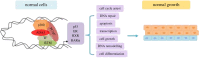
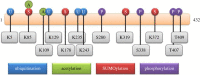
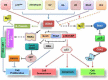
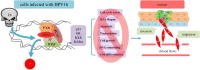
Human ADA3, the evolutionarily conserved transcriptional co-activator, remains the unified part of multiple cellular functions, including regulation of nuclear receptor functions, cell proliferation, apoptosis, senescence, chromatin remodelling, genomic stability and chromosomal maintenance. The past decade has witnessed exciting findings leading to considerable expansion in research related to the biology and regulation of hADA3. Embryonic lethality in homozygous knockout Ada3 mouse signifies the importance of this gene product during early embryonic development. Moreover, the fact that it is a novel target of Human Papillomavirus E6 oncoprotein, one of the most prevalent causal agents behind cervical cancer, helps highlight some of the crucial aspects of HPV-mediated oncogenesis. These findings imply the central involvement of hADA3 in regulation of various cellular functional losses accountable for the genesis of malignancy and viral infections. Recent reports also provide evidence for post-translational modifications of hADA3 leading to its instability and contributing to the malignant phenotype of cervical cancer cells. Furthermore, its association with poor prognosis of breast cancer suggests intimate association in the pathogenesis of the disease. Here, we present the first review on hADA3 with a comprehensive outlook on the molecular and functional roles of hADA3 to provoke further interest for more elegant and intensive studies exploring assorted aspects of this protein.
Keywords: ADA3; cancer; co-activator; development; genomic stability.
© 2016 The Authors.
Figures

References
-
- Berger SL, Piña B, Silverman N, Marcus GA, Agapite J, Regier JL, Triezenberg SJ, Guarente L. 1992. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell 70, 251–265. (doi:10.1016/0092-8674(92)90100-Q) - DOI - PubMed
-
- Pina B, Berger S, Marcus GA, Silverman N, Agapite J, Guarente L. 1993. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol. Cell Biol. 13, 5981–5989. (doi:10.1128/MCB.13.10.5981) - DOI - PMC - PubMed
-
- Ogryzko VV, Kotani T, Zhang X, Schiltz RL, Howard T, Yang X-J, Howard BH, Qin J, Nakatani Y. 1998. Histone-like TAFs within the PCAF histone acetylase complex. Cell 94, 35–44. (doi:10.1016/S0092-8674(00)81219-2) - DOI - PubMed
-
- Syntichaki P, Thireos G. 1998. The Gcn5.Ada complex potentiates the histone acetyltransferase activity of Gcn5. J. Biol. Chem. 273, 24 414–24 419. (doi:10.1074/jbc.273.38.24414) - DOI - PubMed
-
- Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S. 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277, 7989–7995. (doi:10.1074/jbc.M110849200) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
