Editor's Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues
- PMID: 27605418
- PMCID: PMC5139067
- DOI: 10.1093/toxsci/kfw169
Editor's Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues
Abstract
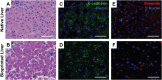
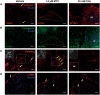
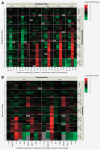
Compound-induced liver injury leading to fibrosis remains a challenge for the development of an Adverse Outcome Pathway useful for human risk assessment. Latency to detection and lack of early, systematically detectable biomarkers make it difficult to characterize the dynamic and complex intercellular interactions that occur during progressive liver injury. Here, we demonstrate the utility of bioprinted tissue constructs comprising primary hepatocytes, hepatic stellate cells, and endothelial cells to model methotrexate- and thioacetamide-induced liver injury leading to fibrosis. Repeated, low-concentration exposure to these compounds enabled the detection and differentiation of multiple modes of liver injury, including hepatocellular damage, and progressive fibrogenesis characterized by the deposition and accumulation of fibrillar collagens in patterns analogous to those described in clinical samples obtained from patients with fibrotic liver injury. Transient cytokine production and upregulation of fibrosis-associated genes ACTA2 and COL1A1 mimics hallmark features of a classic wound-healing response. A surge in proinflammatory cytokines (eg, IL-8, IL-1β) during the early culture time period is followed by concentration- and treatment-dependent alterations in immunomodulatory and chemotactic cytokines such as IL-13, IL-6, and MCP-1. These combined data provide strong proof-of-concept that 3D bioprinted liver tissues can recapitulate drug-, chemical-, and TGF-β1-induced fibrogenesis at the cellular, molecular, and histological levels and underscore the value of the model for further exploration of compound-specific fibrogenic responses. This novel system will enable a more comprehensive characterization of key attributes unique to fibrogenic agents during the onset and progression of liver injury as well as mechanistic insights, thus improving compound risk assessment.
Keywords: 3D bioprinted liver; compound-induced liver injury; liver fibrosis in vitro.
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice.Gastroenterology. 2012 Mar;142(3):612-621.e5. doi: 10.1053/j.gastro.2011.11.029. Epub 2011 Dec 1. Gastroenterology. 2012. PMID: 22138190 Free PMC article.
-
Bioprinted liver provides early insight into the role of Kupffer cells in TGF-β1 and methotrexate-induced fibrogenesis.PLoS One. 2019 Jan 2;14(1):e0208958. doi: 10.1371/journal.pone.0208958. eCollection 2019. PLoS One. 2019. PMID: 30601836 Free PMC article.
-
Detrimental effects of nicotine on thioacetamide-induced liver injury in mice.Toxicol Mech Methods. 2017 Sep;27(7):501-510. doi: 10.1080/15376516.2017.1323256. Epub 2017 May 16. Toxicol Mech Methods. 2017. PMID: 28440100
-
Mechanisms of hepatic fibrogenesis.Best Pract Res Clin Gastroenterol. 2011 Apr;25(2):195-206. doi: 10.1016/j.bpg.2011.02.005. Best Pract Res Clin Gastroenterol. 2011. PMID: 21497738 Free PMC article. Review.
-
Management strategies for liver fibrosis.Ann Hepatol. 2017 Jan-Feb;16(1):48-56. doi: 10.5604/16652681.1226814. Ann Hepatol. 2017. PMID: 28051792 Review.
Cited by
-
Engineering Modular 3D Liver Culture Microenvironments In Vitro to Parse the Interplay between Biophysical and Biochemical Microenvironment Cues on Hepatic Phenotypes.Adv Nanobiomed Res. 2022 Jan;2(1):2100049. doi: 10.1002/anbr.202100049. Epub 2021 Nov 19. Adv Nanobiomed Res. 2022. PMID: 35872804 Free PMC article.
-
Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies.Cells. 2020 Jan 27;9(2):304. doi: 10.3390/cells9020304. Cells. 2020. PMID: 32012725 Free PMC article. Review.
-
Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives.Cells. 2023 Aug 22;12(17):2129. doi: 10.3390/cells12172129. Cells. 2023. PMID: 37681858 Free PMC article. Review.
-
Microphysiological systems in absorption, distribution, metabolism, and elimination sciences.Clin Transl Sci. 2022 Jan;15(1):9-42. doi: 10.1111/cts.13132. Epub 2021 Aug 26. Clin Transl Sci. 2022. PMID: 34378335 Free PMC article. Review.
-
3D Humanized Bioprinted Tubulointerstitium Model to Emulate Renal Fibrosis In Vitro.Adv Healthc Mater. 2024 Nov;13(29):e2400807. doi: 10.1002/adhm.202400807. Epub 2024 Aug 17. Adv Healthc Mater. 2024. PMID: 39152919 Free PMC article.
References
-
- Ankley G. T., Bennett R. S., Erickson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K., et al. (2010). Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. 10.1002/etc.34. - PubMed
-
- Attallah A. M., Mosa T. E., Omran M. M., Abo-Zeid M. M., El-Dosoky I., Shaker Y. M. (2007). Immunodetection of collagen types I, II, III, and IV for differentiation of liver fibrosis stages in patients with chronic HCV. J. Immunoassay Immunochem. 28, 155–168. 10.1080/15321810701212088. - PubMed
-
- Baeck C., Wehr A., Karlmark K. R., Heymann F., Vucur M., Gassler N., Huss S., Klussmann S., Eulberg D., Luedde T., et al. (2012). Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61, 416–426. 10.1136/gutjnl-2011-300304. - PubMed
-
- Barker J., Horn E. J., Lebwohl M., Warren R. B., Nast A., Rosenberg W., Smith C., International Psoriasis C. (2011). Assessment and management of methotrexate hepatotoxicity in psoriasis patients: report from a consensus conference to evaluate current practice and identify key questions toward optimizing methotrexate use in the clinic. J. Eur. Acad. Dermatol. Venereol. 25, 758–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

