Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82
- PMID: 27605431
- PMCID: PMC5015098
- DOI: 10.1038/srep32926
Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82
Abstract
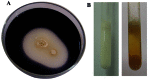
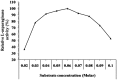
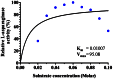
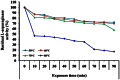
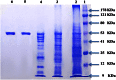
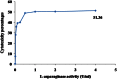
L-asparaginase is an important enzyme as therapeutic agents used in combination with other drugs in the treatment of acute lymphoblastic leukemia. A newly isolated actinomycetes strain, Streptomyces sp. NEAE-82, was potentially producing extracellular L-asparaginase, it was identified as Streptomyces fradiae NEAE-82, sequencing product was deposited in the GenBank database under accession number KJ467538. L-asparaginase was purified from the crude enzyme using ammonium sulfate precipitation, dialysis and ion exchange chromatography using DEAE Sepharose CL-6B. Further the kinetic studies of purified enzyme were carried out. The optimum pH, temperature and incubation time for maximum L-asparaginase activity were found to be 8.5, 40 °C and 30 min, respectively. The optimum substrate concentration was found to be 0.06 M. The Km and Vmax of the enzyme were 0.01007 M and 95.08 Uml(-1)min(-1), respectively. The half-life time (T1/2) was 184.91 min at 50 °С, while being 179.53 min at 60 °С. The molecular weight of the subunits of L-asparaginase was found to be approximately 53 kDa by SDS-PAGE analysis. The purified L-asparaginase showed a final specific activity of 30.636 U/mg protein and was purified 3.338-fold. The present work for the first time reported more information in the production, purification and characterization of L-asparaginase produced by newly isolated actinomycetes Streptomyces fradiae NEAE-82.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









Similar articles
-
Purification, characterization and immunogenicity assessment of glutaminase free L-asparaginase from Streptomyces brollosae NEAE-115.BMC Pharmacol Toxicol. 2018 Aug 23;19(1):51. doi: 10.1186/s40360-018-0242-1. BMC Pharmacol Toxicol. 2018. PMID: 30139388 Free PMC article.
-
Purification, characterization and amino acid content of cholesterol oxidase produced by Streptomyces aegyptia NEAE 102.BMC Microbiol. 2017 Mar 29;17(1):76. doi: 10.1186/s12866-017-0988-4. BMC Microbiol. 2017. PMID: 28356065 Free PMC article.
-
Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines.Sci Rep. 2020 May 14;10(1):7942. doi: 10.1038/s41598-020-64052-x. Sci Rep. 2020. PMID: 32409719 Free PMC article.
-
L-asparaginase: a promising chemotherapeutic agent.Crit Rev Biotechnol. 2007 Jan-Mar;27(1):45-62. doi: 10.1080/07388550601173926. Crit Rev Biotechnol. 2007. PMID: 17364689 Review.
-
Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia.Crit Rev Oncol Hematol. 2007 Mar;61(3):208-21. doi: 10.1016/j.critrevonc.2006.07.009. Epub 2006 Oct 2. Crit Rev Oncol Hematol. 2007. PMID: 17011787 Review.
Cited by
-
Bioprospecting of the agaricomycete Ganoderma australe GPC191 as novel source for L-asparaginase production.Sci Rep. 2021 Mar 18;11(1):6192. doi: 10.1038/s41598-021-84949-5. Sci Rep. 2021. PMID: 33737513 Free PMC article.
-
Microbes Producing L-Asparaginase free of Glutaminase and Urease isolated from Extreme Locations of Antarctic Soil and Moss.Sci Rep. 2019 Feb 5;9(1):1423. doi: 10.1038/s41598-018-38094-1. Sci Rep. 2019. PMID: 30723240 Free PMC article.
-
Metagenomic discovery and functional validation of L-asparaginases with anti-leukemic effect from the Caspian Sea.iScience. 2021 Jan 5;24(1):101973. doi: 10.1016/j.isci.2020.101973. eCollection 2021 Jan 22. iScience. 2021. PMID: 33458619 Free PMC article.
-
Identification of L-asparaginases from Streptomyces strains with competitive activity and immunogenic profiles: a bioinformatic approach.PeerJ. 2020 Nov 13;8:e10276. doi: 10.7717/peerj.10276. eCollection 2020. PeerJ. 2020. PMID: 33240625 Free PMC article.
-
Optimizing Gene Sources for L-asparaginase Production: A Comparative Review.Curr Microbiol. 2025 Jul 12;82(9):381. doi: 10.1007/s00284-025-04351-6. Curr Microbiol. 2025. PMID: 40646362 Review.
References
-
- Verma N., Kumar K., Kaur G. & Anand S. E. coli K-12 asparaginase-based asparagine biosensor for leukemia. Artif Cells Blood Substit Immobil Biotechnol 35, 449–456 (2007). - PubMed
-
- Pedreschi F., Kaack K. & Granby K. The effect of asparaginase on acrylamide formation in French fries. Food chem 109, 386–392 (2008). - PubMed
-
- Duval M. et al.. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood 99, 2734–2739 (2002). - PubMed
-
- Kotzia G. A. & Lbrou N. E. Cloning, expression and characterisation of Erwinia carotovora L-asparaginase. J Biotechnol 119, 309–323 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

