Buttressing a balanced brain: Target-derived FGF signaling regulates excitatory/inhibitory tone and adult neurogenesis within the maturating hippocampal network
- PMID: 27605441
- PMCID: PMC4973582
- DOI: 10.1080/23262133.2016.1168504
Buttressing a balanced brain: Target-derived FGF signaling regulates excitatory/inhibitory tone and adult neurogenesis within the maturating hippocampal network
Abstract
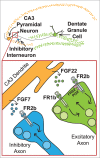
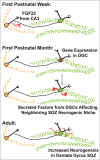
Brain development involves multiple levels of molecular coordination in forming a functional nervous system. The hippocampus is a brain area that is important for memory formation and spatial reasoning. During early postnatal development of the hippocampal circuit, Fibroblast growth factor 22 (FGF22) and FGF7 act to establish a balance of excitatory and inhibitory tone. Both FGFs are secreted from CA3 dendrites, acting on excitatory or inhibitory axon terminals formed onto CA3 dendrites, respectively. Mechanistically, FGF22 utilizes FGFR2b and FGFR1b to induce synaptic vesicle recruitment within axons of dentate granule cells (DGCs), and FGF7 utilizes FGFR2b to induce synaptic vesicle recruitment within interneuron axons. FGF signaling eventually induces gene expression in the presynaptic neurons; however, the effects of FGF22-induced gene expression within DGCs and FGF7-induced gene expression within interneurons in the context of a developing hippocampal circuit have yet to be explored. Here, we propose one hypothetical mechanism of FGF22-induced gene expression in controlling adult neurogenesis.
Keywords: FGF22; FGF7; FGFR1b; FGFR2b; Fibroblast growth factor receptors; adult neurogenesis; excitatory/inhibitory; hippocampus; presynaptic differentiation; synapse development.
Figures
Comment on
-
Distinct sets of FGF receptors sculpt excitatory and inhibitory synaptogenesis.Development. 2015 May 15;142(10):1818-30. doi: 10.1242/dev.115568. Epub 2015 Apr 29. Development. 2015. PMID: 25926357 Free PMC article.
References
-
- Dabrowski A, Terauchi A, Strong C, Umemori H. Distinct sets of FGF receptors sculpt excitatory and inhibitory synaptogenesis. Development 2015; 142:1818-30; PMID:25926357; http://dx.doi.org/10.1242/dev.115568 - DOI - PMC - PubMed
-
- Basu J, Siegelbaum SA. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb Perspect Biol 2015; 7: pii: a021733; PMID:26525152; http://dx.doi.org/10.1101/cshperspect.a021733 - DOI - PMC - PubMed
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8:45-56; PMID:17180162; http://dx.doi.org/10.1038/nrn2044 - DOI - PubMed
-
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol 2015; 7:a018812; PMID:26330519; http://dx.doi.org/10.1101/cshperspect.a018812 - DOI - PMC - PubMed
-
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med 2008; 10:128-40; PMID:18286389; http://dx.doi.org/10.1007/s12017-008-8028-z - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
