Prefrontal Neurons Represent Motion Signals from Across the Visual Field But for Memory-Guided Comparisons Depend on Neurons Providing These Signals
- PMID: 27605611
- PMCID: PMC5013185
- DOI: 10.1523/JNEUROSCI.0843-16.2016
Prefrontal Neurons Represent Motion Signals from Across the Visual Field But for Memory-Guided Comparisons Depend on Neurons Providing These Signals
Abstract
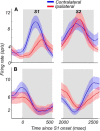
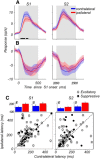
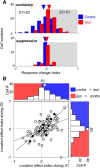
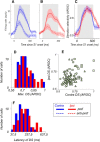
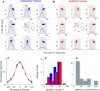
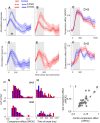
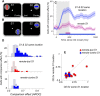
Visual decisions often involve comparisons of sequential stimuli that can appear at any location in the visual field. The lateral prefrontal cortex (LPFC) in nonhuman primates, shown to play an important role in such comparisons, receives information about contralateral stimuli directly from sensory neurons in the same hemisphere, and about ipsilateral stimuli indirectly from neurons in the opposite hemisphere. This asymmetry of sensory inputs into the LPFC poses the question of whether and how its neurons incorporate sensory information arriving from the two hemispheres during memory-guided comparisons of visual motion. We found that, although responses of individual LPFC neurons to contralateral stimuli were stronger and emerged 40 ms earlier, they carried remarkably similar signals about motion direction in the two hemifields, with comparable direction selectivity and similar direction preferences. This similarity was also apparent around the time of the comparison between the current and remembered stimulus because both ipsilateral and contralateral responses showed similar signals reflecting the remembered direction. However, despite availability in the LPFC of motion information from across the visual field, these "comparison effects" required for the comparison stimuli to appear at the same retinal location. This strict dependence on spatial overlap of the comparison stimuli suggests participation of neurons with localized receptive fields in the comparison process. These results suggest that while LPFC incorporates many key aspects of the information arriving from sensory neurons residing in opposite hemispheres, it continues relying on the interactions with these neurons at the time of generating signals leading to successful perceptual decisions.
Significance statement: Visual decisions often involve comparisons of sequential visual motion that can appear at any location in the visual field. We show that during such comparisons, the lateral prefrontal cortex (LPFC) contains accurate representation of visual motion from across the visual field, supplied by motion processing neurons. However, at the time of comparison, LPFC neurons can only use this information to compute the differences between the stimuli, if stimuli appear at the same retinal location, implicating neurons with localized receptive fields in the comparison process. These findings show that sensory comparisons rely on the interactions between LPFC and sensory neurons that not only supply sensory signals but also actively participate in the comparison of these signals at the time of the decision.
Keywords: direction selectivity; hemifields; working memory.
Copyright © 2016 the authors 0270-6474/16/369351-14$15.00/0.
Figures
Similar articles
-
Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits.J Neurosci. 2016 Jan 13;36(2):489-505. doi: 10.1523/JNEUROSCI.3678-15.2016. J Neurosci. 2016. PMID: 26758840 Free PMC article.
-
Unilateral prefrontal lesions impair memory-guided comparisons of contralateral visual motion.J Neurosci. 2015 May 6;35(18):7095-105. doi: 10.1523/JNEUROSCI.5265-14.2015. J Neurosci. 2015. PMID: 25948260 Free PMC article.
-
Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task.J Neurosci. 2006 Nov 8;26(45):11726-42. doi: 10.1523/JNEUROSCI.3420-06.2006. J Neurosci. 2006. PMID: 17093094 Free PMC article.
-
Linking Neuronal Direction Selectivity to Perceptual Decisions About Visual Motion.Annu Rev Vis Sci. 2020 Sep 15;6:335-362. doi: 10.1146/annurev-vision-121219-081816. Annu Rev Vis Sci. 2020. PMID: 32936737 Review.
-
Does experience provide a permissive or instructive influence on the development of direction selectivity in visual cortex?Neural Dev. 2018 Jul 12;13(1):16. doi: 10.1186/s13064-018-0113-x. Neural Dev. 2018. PMID: 30001203 Free PMC article. Review.
Cited by
-
Neural Representation of Motor Output, Context and Behavioral Adaptation in Rat Medial Prefrontal Cortex During Learned Behavior.Front Neural Circuits. 2018 Oct 1;12:75. doi: 10.3389/fncir.2018.00075. eCollection 2018. Front Neural Circuits. 2018. PMID: 30327591 Free PMC article.
-
Interhemispheric transfer of working memories.Neuron. 2021 Mar 17;109(6):1055-1066.e4. doi: 10.1016/j.neuron.2021.01.016. Epub 2021 Feb 8. Neuron. 2021. PMID: 33561399 Free PMC article.
-
A Normalization Circuit Underlying Coding of Spatial Attention in Primate Lateral Prefrontal Cortex.eNeuro. 2019 Apr 15;6(2):ENEURO.0301-18.2019. doi: 10.1523/ENEURO.0301-18.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 31001577 Free PMC article.
-
Distractor anticipation during working memory is associated with theta and beta oscillations across spatial scales.Front Integr Neurosci. 2025 Mar 24;19:1553521. doi: 10.3389/fnint.2025.1553521. eCollection 2025. Front Integr Neurosci. 2025. PMID: 40196759 Free PMC article.
-
Working memory expedites the processing of visual signals within the extrastriate cortex.iScience. 2024 Jul 11;27(8):110489. doi: 10.1016/j.isci.2024.110489. eCollection 2024 Aug 16. iScience. 2024. PMID: 39100691 Free PMC article.
References
-
- Boch RA, Goldberg ME. Participation of prefrontal neurons in the preparation of visually guided eye movements in the rhesus monkey. J Neurophysiol. 1989;61:1064–1084. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
