A Corticocortical Circuit Directly Links Retrosplenial Cortex to M2 in the Mouse
- PMID: 27605612
- PMCID: PMC5013186
- DOI: 10.1523/JNEUROSCI.1099-16.2016
A Corticocortical Circuit Directly Links Retrosplenial Cortex to M2 in the Mouse
Abstract
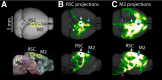
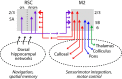
Retrosplenial cortex (RSC) is a dorsomedial parietal area involved in a range of cognitive functions, including episodic memory, navigation, and spatial memory. Anatomically, the RSC receives inputs from dorsal hippocampal networks and in turn projects to medial neocortical areas. A particularly prominent projection extends rostrally to the posterior secondary motor cortex (M2), suggesting a functional corticocortical link from the RSC to M2 and thus a bridge between hippocampal and neocortical networks involved in mnemonic and sensorimotor aspects of navigation. We investigated the cellular connectivity in this RSC→M2 projection in the mouse using optogenetic photostimulation, retrograde labeling, and electrophysiology. Axons from RSC formed monosynaptic excitatory connections onto M2 pyramidal neurons across layers and projection classes, including corticocortical/intratelencephalic neurons (reciprocally and callosally projecting) in layers 2-6, pyramidal tract neurons (corticocollicular, corticopontine) in layer 5B, and, to a lesser extent, corticothalamic neurons in layer 6. In addition to these direct connections, disynaptic connections were made via posterior parietal cortex (RSC→PPC→M2) and anteromedial thalamus (RSC→AM→M2). In the reverse direction, axons from M2 monosynaptically excited M2-projecting corticocortical neurons in the RSC, especially in the superficial layers of the dysgranular region. These findings establish an excitatory RSC→M2 corticocortical circuit that engages diverse types of excitatory projection neurons in the downstream area, suggesting a basis for direct communication from dorsal hippocampal networks involved in spatial memory and navigation to neocortical networks involved in diverse aspects of sensorimotor integration and motor control.
Significance statement: Corticocortical pathways interconnect cortical areas extensively, but the cellular connectivity in these pathways remains largely uncharacterized. Here, we show that a posterior part of secondary motor cortex receives corticocortical axons from the rostral retrosplenial cortex (RSC) and these form monosynaptic excitatory connections onto a wide spectrum of excitatory projection neurons in this area. Our results define a cellular basis for direct communication from RSC to this medial frontal area, suggesting a direct link from dorsal hippocampal networks involved in spatial cognition and navigation (the "map") to sensorimotor networks involved the control of movement (the "motor").
Keywords: circuit; motor; neocortex; optogenetic; retrosplenial.
Copyright © 2016 the authors 0270-6474/16/369365-10$15.00/0.
Figures







Similar articles
-
Anterolateral Motor Cortex Connects with a Medial Subdivision of Ventromedial Thalamus through Cell Type-Specific Circuits, Forming an Excitatory Thalamo-Cortico-Thalamic Loop via Layer 1 Apical Tuft Dendrites of Layer 5B Pyramidal Tract Type Neurons.J Neurosci. 2018 Oct 10;38(41):8787-8797. doi: 10.1523/JNEUROSCI.1333-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143573 Free PMC article.
-
Projection-specific circuits of retrosplenial cortex with differential contributions to spatial cognition.Mol Psychiatry. 2025 May;30(5):2068-2084. doi: 10.1038/s41380-024-02819-8. Epub 2024 Nov 7. Mol Psychiatry. 2025. PMID: 39511453 Free PMC article.
-
Reciprocal interareal connections to corticospinal neurons in mouse M1 and S2.J Neurosci. 2015 Feb 18;35(7):2959-74. doi: 10.1523/JNEUROSCI.4287-14.2015. J Neurosci. 2015. PMID: 25698734 Free PMC article.
-
Local connections of excitatory neurons in motor-associated cortical areas of the rat.Front Neural Circuits. 2013 May 28;7:75. doi: 10.3389/fncir.2013.00075. eCollection 2013. Front Neural Circuits. 2013. PMID: 23754982 Free PMC article. Review.
-
Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition.Front Hum Neurosci. 2014 Aug 5;8:586. doi: 10.3389/fnhum.2014.00586. eCollection 2014. Front Hum Neurosci. 2014. PMID: 25140141 Free PMC article. Review.
Cited by
-
Reconstruction of Intratelencephalic Neurons in the Mouse Secondary Motor Cortex Reveals the Diverse Projection Patterns of Single Neurons.Front Neuroanat. 2018 Oct 30;12:86. doi: 10.3389/fnana.2018.00086. eCollection 2018. Front Neuroanat. 2018. PMID: 30425624 Free PMC article.
-
Scaling of Optogenetically Evoked Signaling in a Higher-Order Corticocortical Pathway in the Anesthetized Mouse.Front Syst Neurosci. 2018 May 15;12:16. doi: 10.3389/fnsys.2018.00016. eCollection 2018. Front Syst Neurosci. 2018. PMID: 29867381 Free PMC article.
-
Cortical Observation by Synchronous Multifocal Optical Sampling Reveals Widespread Population Encoding of Actions.Neuron. 2020 Jul 22;107(2):351-367.e19. doi: 10.1016/j.neuron.2020.04.023. Epub 2020 May 19. Neuron. 2020. PMID: 32433908 Free PMC article.
-
The fully automated bat (FAB) flight room: A human-free environment for studying navigation in flying bats and its initial application to the retrosplenial cortex.J Neurosci Methods. 2021 Jan 15;348:108970. doi: 10.1016/j.jneumeth.2020.108970. Epub 2020 Oct 14. J Neurosci Methods. 2021. PMID: 33065152 Free PMC article.
-
FOS mapping reveals two complementary circuits for spatial navigation in mouse.Sci Rep. 2024 Sep 11;14(1):21252. doi: 10.1038/s41598-024-72272-8. Sci Rep. 2024. PMID: 39261637 Free PMC article.
References
-
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004;479:360–373. doi: 10.1002/cne.20306. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
