Pten Regulates Retinal Amacrine Cell Number by Modulating Akt, Tgfβ, and Erk Signaling
- PMID: 27605619
- PMCID: PMC6601873
- DOI: 10.1523/JNEUROSCI.0936-16.2016
Pten Regulates Retinal Amacrine Cell Number by Modulating Akt, Tgfβ, and Erk Signaling
Abstract
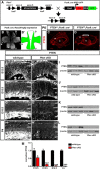
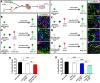
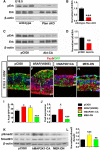
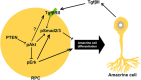
All tissues are genetically programmed to acquire an optimal size that is defined by total cell number and individual cellular dimensions. The retina contains stereotyped proportions of one glial and six neuronal cell types that are generated in overlapping waves. How multipotent retinal progenitors know when to switch from making one cell type to the next so that appropriate numbers of each cell type are generated is poorly understood. Pten is a phosphatase that controls progenitor cell proliferation and differentiation in several lineages. Here, using a conditional loss-of-function strategy, we found that Pten regulates retinal cell division and is required to produce the full complement of rod photoreceptors and amacrine cells in mouse. We focused on amacrine cell number control, identifying three downstream Pten effector pathways. First, phosphoinositide 3-kinase/Akt signaling is hyperactivated in Pten conditional knock-out (cKO) retinas, and misexpression of constitutively active Akt (Akt-CA) in retinal explants phenocopies the reduction in amacrine cell production observed in Pten cKOs. Second, Akt-CA activates Tgfβ signaling in retinal explants, which is a negative feedback pathway for amacrine cell production. Accordingly, Tgfβ signaling is elevated in Pten cKO retinas, and epistatic analyses placed Pten downstream of TgfβRII in amacrine cell number control. Finally, Pten regulates Raf/Mek/Erk signaling levels to promote the differentiation of all amacrine cell subtypes, which are each reduced in number in Pten cKOs. Pten is thus a positive regulator of amacrine cell production, acting via multiple downstream pathways, highlighting its diverse actions as a mediator of cell number control.
Significance statement: Despite the importance of size for optimal organ function, how individual cell types are generated in correct proportions is poorly understood. There are several ways to control cell number, including readouts of organ function (e.g., secreted hormones reach functional levels when enough cells are made) or counting of cell divisions or cell number. The latter applies to the retina, where cell number is regulated by negative feedback signals, which arrest differentiation of particular cell types at threshold levels. Herein, we show that Pten is a critical regulator of amacrine cell number in the retina, acting via multiple downstream pathways. Our studies provide molecular insights into how PTEN loss in humans may lead to uncontrolled cell division in several pathological conditions.
Keywords: Erk; Pten; Tgfβ; amacrine cells; negative feedback signaling; retina.
Copyright © 2016 the authors 0270-6474/16/369454-18$15.00/0.
Figures







Similar articles
-
Zac1 functions through TGFbetaII to negatively regulate cell number in the developing retina.Neural Dev. 2007 Jun 8;2:11. doi: 10.1186/1749-8104-2-11. Neural Dev. 2007. PMID: 17559664 Free PMC article.
-
PTEN regulates retinal interneuron morphogenesis and synaptic layer formation.Mol Cell Neurosci. 2012 Feb;49(2):171-83. doi: 10.1016/j.mcn.2011.11.007. Epub 2011 Dec 4. Mol Cell Neurosci. 2012. PMID: 22155156 Free PMC article.
-
Cell-type specific roles for PTEN in establishing a functional retinal architecture.PLoS One. 2012;7(3):e32795. doi: 10.1371/journal.pone.0032795. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403711 Free PMC article.
-
Cell numbers, cell ratios, and developmental plasticity in the rod pathway of the mouse retina.J Anat. 2023 Aug;243(2):204-222. doi: 10.1111/joa.13653. Epub 2022 Mar 15. J Anat. 2023. PMID: 35292986 Free PMC article. Review.
-
The Lgr5 transgene is expressed specifically in glycinergic amacrine cells in the mouse retina.Exp Eye Res. 2014 Feb;119:106-10. doi: 10.1016/j.exer.2013.11.003. Epub 2013 Nov 15. Exp Eye Res. 2014. PMID: 24246263 Free PMC article. Review.
Cited by
-
Regulation of retinal amacrine cell generation by miR-216b and Foxn3.Development. 2022 Jan 15;149(2):dev199484. doi: 10.1242/dev.199484. Epub 2022 Jan 17. Development. 2022. PMID: 34919141 Free PMC article.
-
Pten regulates endocytic trafficking of cell adhesion and Wnt signaling molecules to pattern the retina.Cell Rep. 2024 Apr 23;43(4):114005. doi: 10.1016/j.celrep.2024.114005. Epub 2024 Mar 27. Cell Rep. 2024. PMID: 38551961 Free PMC article.
-
PTEN Expression Regulates Gap Junction Connectivity in the Retina.Front Neuroanat. 2021 May 20;15:629244. doi: 10.3389/fnana.2021.629244. eCollection 2021. Front Neuroanat. 2021. PMID: 34093139 Free PMC article.
-
Intracellular Invasion and Killing Assay to Investigate the Effectsof Binge Alcohol Toxicity in Murine Alveolar Macrophages.Bio Protoc. 2019 Jan 20;9(2):e3143. doi: 10.21769/BioProtoc.3143. eCollection 2019 Jan 20. Bio Protoc. 2019. PMID: 33654888 Free PMC article.
-
Beyond Genetics: The Role of Metabolism in Photoreceptor Survival, Development and Repair.Front Cell Dev Biol. 2022 May 18;10:887764. doi: 10.3389/fcell.2022.887764. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35663397 Free PMC article. Review.
References
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
