Topical Prostaglandin E Analog Restores Defective Dendritic Cell-Mediated Th17 Host Defense Against Methicillin-Resistant Staphylococcus Aureus in the Skin of Diabetic Mice
- PMID: 27605625
- PMCID: PMC5127243
- DOI: 10.2337/db16-0565
Topical Prostaglandin E Analog Restores Defective Dendritic Cell-Mediated Th17 Host Defense Against Methicillin-Resistant Staphylococcus Aureus in the Skin of Diabetic Mice
Abstract
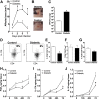
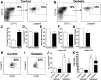
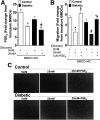
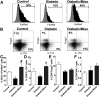
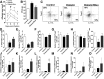
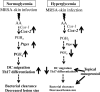
People with diabetes are more prone to Staphylococcus aureus skin infection than healthy individuals. Control of S. aureus infection depends on dendritic cell (DC)-induced T-helper 17 (Th17)-mediated neutrophil recruitment and bacterial clearance. DC ingestion of infected apoptotic cells (IACs) drive prostaglandin E2 (PGE2) secretion to generate Th17 cells. We speculated that hyperglycemia inhibits skin DC migration to the lymph nodes and impairs the Th17 differentiation that accounts for poor skin host defense in diabetic mice. Diabetic mice showed increased skin lesion size and bacterial load and decreased PGE2 secretion and Th17 cells compared with nondiabetic mice after methicillin-resistant S. aureus (MRSA) infection. Bone marrow-derived DCs (BMDCs) cultured in high glucose (25 mmol/L) exhibited decreased Ptges mRNA expression, PGE2 production, lower CCR7-dependent DC migration, and diminished maturation after recognition of MRSA-IACs than BMDCs cultured in low glucose (5 mmol/L). Similar events were observed in DCs from diabetic mice infected with MRSA. Topical treatment of diabetic mice with the PGE analog misoprostol improved host defense against MRSA skin infection by restoring DC migration to draining lymph nodes, Th17 differentiation, and increased antimicrobial peptide expression. These findings identify a novel mechanism involved in poor skin host defense in diabetes and propose a targeted strategy to restore skin host defense in diabetes.
© 2016 by the American Diabetes Association.
Figures


Similar articles
-
PSM Peptides From Community-Associated Methicillin-Resistant Staphylococcus aureus Impair the Adaptive Immune Response via Modulation of Dendritic Cell Subsets in vivo.Front Immunol. 2019 May 10;10:995. doi: 10.3389/fimmu.2019.00995. eCollection 2019. Front Immunol. 2019. PMID: 31134074 Free PMC article.
-
Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice.JCI Insight. 2018 Sep 6;3(17):e120220. doi: 10.1172/jci.insight.120220. eCollection 2018 Sep 6. JCI Insight. 2018. PMID: 30185672 Free PMC article.
-
Cholera Toxin Promotes Th17 Cell Differentiation by Modulating Expression of Polarizing Cytokines and the Antigen-Presenting Potential of Dendritic Cells.PLoS One. 2016 Jun 6;11(6):e0157015. doi: 10.1371/journal.pone.0157015. eCollection 2016. PLoS One. 2016. PMID: 27271559 Free PMC article.
-
Role of cytokines in host defense against Staphylococcus aureus skin infection.Histol Histopathol. 2017 Aug;32(8):761-766. doi: 10.14670/HH-11-867. Epub 2017 Jan 12. Histol Histopathol. 2017. PMID: 28078661 Review.
-
Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides.Int J Mol Sci. 2014 May 16;15(5):8753-72. doi: 10.3390/ijms15058753. Int J Mol Sci. 2014. PMID: 24840573 Free PMC article. Review.
Cited by
-
Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes.Prostaglandins Other Lipid Mediat. 2020 Apr;147:106398. doi: 10.1016/j.prostaglandins.2019.106398. Epub 2019 Nov 11. Prostaglandins Other Lipid Mediat. 2020. PMID: 31726221 Free PMC article.
-
Intestinal host defense outcome is dictated by PGE2 production during efferocytosis of infected cells.Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):E8469-E8478. doi: 10.1073/pnas.1722016115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127026 Free PMC article.
-
Staphylococcus xylosus and Staphylococcus aureus as commensals and pathogens on murine skin.Lab Anim Res. 2023 Aug 2;39(1):18. doi: 10.1186/s42826-023-00169-0. Lab Anim Res. 2023. PMID: 37533118 Free PMC article. Review.
-
TXA2 attenuates allergic lung inflammation through regulation of Th2, Th9, and Treg differentiation.J Clin Invest. 2024 Mar 14;134(9):e165689. doi: 10.1172/JCI165689. J Clin Invest. 2024. PMID: 38483511 Free PMC article.
-
PD-L1 Reverse Signaling in Dermal Dendritic Cells Promotes Dendritic Cell Migration Required for Skin Immunity.Cell Rep. 2020 Oct 13;33(2):108258. doi: 10.1016/j.celrep.2020.108258. Cell Rep. 2020. PMID: 33053342 Free PMC article.
References
-
- Muller LMAJ, Gorter KJ, Hak E, et al. . Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005;41:281–288 - PubMed
-
- Dryden M, Baguneid M, Eckmann C, et al. . Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect 2015;21(Suppl. 2):S27–S32 - PubMed
-
- Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 2007;23:3–13 - PubMed
-
- Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010;65(Suppl. 3):iii35–iii44 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

