Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer
- PMID: 27605871
- PMCID: PMC4968117
- DOI: 10.3748/wjg.v22.i28.6345
Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer
Abstract
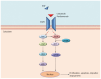
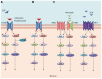
The prognosis of patients with metastatic colorectal cancer (mCRC) remain poor despite the impressive improvement of treatments observed over the last 20 years that led to an increase in median overall survival from 6 mo, with the only best supportive care, to approximately 30 mo with the introduction of active chemotherapy drugs and targeted agents. The monoclonal antibodies (moAbs) cetuximab and panitumumab, directed against the epidermal growth factor receptor (EGFR), undoubtedly represent a major step forward in the treatment of mCRC, given the relevant efficacy in terms of progression-free survival, overall survival, response rate, and quality of life observed in several phase III clinical trials among different lines of treatment. However, the anti-EGFR moAbs were shown only to be effective in a subset of patients. For instance, KRAS and NRAS mutations have been identified as biomarkers of resistance to these drugs, improving the selection of patients who might derive a benefit from these treatments. Nevertheless, several other alterations might affect the response to these drugs, and unfortunately, even the responders eventually become resistant by developing secondary (or acquired) resistance in approximately 13-18 mo. Several studies highlighted that the landscape of responsible alterations of both primary and acquired resistance to anti-EGFR drugs biochemically converge into MEK-ERK and PIK3CA-AKT pathways. In this review, we describe the currently known mechanisms of primary and acquired resistance to anti-EGFR moAbs together with the various strategies evaluated to prevent, overcame or revert them.
Keywords: BRAF; Epidermal growth factor receptor; KRAS; MET; Metastatic colorectal cancer; Monoclonal antibodies; Mutation; NRAS; PIK3CA; Resistance.
Figures
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. - PubMed
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1–iii9. - PubMed
-
- Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

