In vivo Whole-Cell Recordings Combined with Electron Microscopy Reveal Unexpected Morphological and Physiological Properties in the Lateral Nucleus of the Trapezoid Body in the Auditory Brainstem
- PMID: 27605909
- PMCID: PMC4995217
- DOI: 10.3389/fncir.2016.00069
In vivo Whole-Cell Recordings Combined with Electron Microscopy Reveal Unexpected Morphological and Physiological Properties in the Lateral Nucleus of the Trapezoid Body in the Auditory Brainstem
Abstract
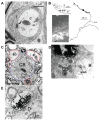
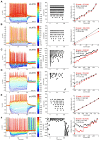
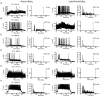
The lateral nucleus of the trapezoid body (LNTB) is a prominent nucleus in the superior olivary complex in mammals including humans. Its physiology in vivo is poorly understood due to a paucity of recordings. It is thought to provide a glycinergic projection to the medial superior olive (MSO) with an important role in binaural processing and sound localization. We combined in vivo patch clamp recordings with labeling of individual neurons in the Mongolian gerbil. Labeling of the recorded neurons allowed us to relate physiological properties to anatomy at the light and electron microscopic level. We identified a population of quite dorsally located neurons with surprisingly large dendritic trees on which most of the synaptic input impinges. In most neurons, one or more of these dendrites run through and are then medial to the MSO. These neurons were often binaural and could even show sensitivity to interaural time differences (ITDs) of stimulus fine structure or envelope. Moreover, a subpopulation showed enhanced phase-locking to tones delivered in the tuning curve tail. We propose that these neurons constitute the gerbil main LNTB (mLNTB). In contrast, a smaller sample of neurons was identified that was located more ventrally and that we designate to be in posteroventral LNTB (pvLNTB). These cells receive large somatic excitatory terminals from globular bushy cells. We also identified previously undescribed synaptic inputs from the lateral superior olive. pvLNTB neurons are usually monaural, display a primary-like-with-notch response to ipsilateral short tones at CF and can phase-lock to low frequency tones. We conclude that mLNTB contains a population of neurons with extended dendritic trees where most of the synaptic input is found, that can show enhanced phase-locking and sensitivity to ITD. pvLNTB cells, presumed to provide glycinergic input to the MSO, get large somatic globular bushy synaptic inputs and are typically monaural with short tone responses similar to their primary input from the cochlear nucleus.
Keywords: ITD; LNTB; LSO; Mongolian gerbil; mLNTB; patch clamp; phase-locking; pvLNTB.
Figures











Similar articles
-
Enhancement of phase-locking in rodents. I. An axonal recording study in gerbil.J Neurophysiol. 2017 Oct 1;118(4):2009-2023. doi: 10.1152/jn.00194.2016. Epub 2017 Jul 12. J Neurophysiol. 2017. PMID: 28701535 Free PMC article.
-
Neurons sensitive to interaural phase disparity in gerbil superior olive: diverse monaural and temporal response properties.J Neurophysiol. 1995 Apr;73(4):1668-90. doi: 10.1152/jn.1995.73.4.1668. J Neurophysiol. 1995. PMID: 7643174
-
Predicting binaural responses from monaural responses in the gerbil medial superior olive.J Neurophysiol. 2016 Jun 1;115(6):2950-63. doi: 10.1152/jn.01146.2015. Epub 2016 Mar 23. J Neurophysiol. 2016. PMID: 27009164 Free PMC article.
-
The function of the medial superior olive in small mammals: temporal receptive fields in auditory analysis.J Comp Physiol A. 2000 May;186(5):413-23. doi: 10.1007/s003590050441. J Comp Physiol A. 2000. PMID: 10879945 Review.
-
Cellular and synaptic specializations for sub-millisecond precision in the mammalian auditory brainstem.Front Cell Neurosci. 2025 May 19;19:1568506. doi: 10.3389/fncel.2025.1568506. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40458470 Free PMC article. Review.
Cited by
-
Glycinergic axonal inhibition subserves acute spatial sensitivity to sudden increases in sound intensity.Elife. 2021 Jun 14;10:e62183. doi: 10.7554/eLife.62183. Elife. 2021. PMID: 34121662 Free PMC article.
-
Cellular Computations Underlying Detection of Gaps in Sounds and Lateralizing Sound Sources.Trends Neurosci. 2017 Oct;40(10):613-624. doi: 10.1016/j.tins.2017.08.001. Epub 2017 Aug 31. Trends Neurosci. 2017. PMID: 28867348 Free PMC article. Review.
-
In Vitro Wedge Slice Preparation for Mimicking In Vivo Neuronal Circuit Connectivity.J Vis Exp. 2020 Aug 18;(162):10.3791/61664. doi: 10.3791/61664. J Vis Exp. 2020. PMID: 32894269 Free PMC article.
-
Neuronal sensitivity to the interaural time difference of the sound envelope in the mouse inferior colliculus.Hear Res. 2020 Jan;385:107844. doi: 10.1016/j.heares.2019.107844. Epub 2019 Nov 15. Hear Res. 2020. PMID: 31759235 Free PMC article.
-
Principal cells of the brainstem's interaural sound level detector are temporal differentiators rather than integrators.Elife. 2018 Jun 14;7:e33854. doi: 10.7554/eLife.33854. Elife. 2018. PMID: 29901438 Free PMC article.
References
-
- Batra R., Kuwada S., Fitzpatrick D. C. (1997). Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. II. Coincidence detection. J. Neurophysiol. 78 1237–1247. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

