Convergence and Divergence of Signaling Events in Guard Cells during Stomatal Closure by Plant Hormones or Microbial Elicitors
- PMID: 27605934
- PMCID: PMC4996035
- DOI: 10.3389/fpls.2016.01332
Convergence and Divergence of Signaling Events in Guard Cells during Stomatal Closure by Plant Hormones or Microbial Elicitors
Abstract
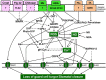
Dynamic regulation of stomatal aperture is essential for plants to optimize water use and CO2 uptake. Stomatal opening or closure is accompanied by the modulation of guard cell turgor. Among the events leading to stomatal closure by plant hormones or microbial elicitors, three signaling components stand out as the major converging points. These are reactive oxygen species (ROS), cytosolic free Ca(2+), and ion channels. Once formed, the ROS and free Ca(2+) of guard cells regulate both downstream and upstream events. A major influence of ROS is to increase the levels of NO and cytosolic free Ca(2+) in guard cells. Although the rise in NO is an important event during stomatal closure, the available evidences do not support the description of NO as the point of convergence. The rise in ROS and NO would cause an increase of free Ca(2+) and modulate ion channels, through a network of events, in such a way that the guard cells lose K(+)/Cl(-)/anions. The efflux of these ions decreases the turgor of guard cells and leads to stomatal closure. Thus, ROS, NO, and cytosolic free Ca(2+) act as points of divergence. The other guard cell components, which are modulated during stomatal closure are G-proteins, cytosolic pH, phospholipids, and sphingolipids. However, the current information on the role of these components is not convincing so as to assign them as the points of convergence or divergence. The interrelationships and interactions of ROS, NO, cytosolic pH, and free Ca(2+) are quite complex and need further detailed examination. Our review is an attempt to critically assess the current status of information on guard cells, while emphasizing the convergence and divergence of signaling components during stomatal closure. The existing gaps in our knowledge are identified to stimulate further research.
Keywords: ABA; ROS; cytosolic free Ca2+; cytosolic pH; guard cells; ion channels; nitric oxide; secondary messengers.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

