Tumor prevention facilitates delayed transplant of stem cell-derived motoneurons
- PMID: 27606345
- PMCID: PMC4999595
- DOI: 10.1002/acn3.327
Tumor prevention facilitates delayed transplant of stem cell-derived motoneurons
Abstract
Objective: Nerve injuries resulting in prolonged periods of denervation result in poor recovery of motor function. We have previously shown that embryonic stem cell-derived motoneurons transplanted at the time of transection into a peripheral nerve can functionally reinnervate muscle. For clinical relevance, we now focused on delaying transplantation to assess reinnervation after prolonged denervation.
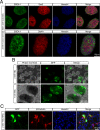
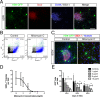
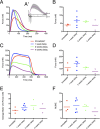
Methods: Embryonic stem cell-derived motoneurons were transplanted into the distal segments of transected tibial nerves in adult mice after prolonged denervation of 1-8 weeks. Twitch and tetanic forces were measured ex vivo 3 months posttransplantation. Tissue was harvested from the transplants for culture and immunohistochemical analysis.
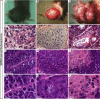
Results: In this delayed reinnervation model, teratocarcinomas developed in about one half of transplants. A residual multipotent cell population (~ 6% of cells) was found despite neural differentiation. Exposure to the alkylating drug mitomycin C eliminated this multipotent population in vitro while preserving motoneurons. Treating neural differentiated stem cells prior to delayed transplantation prevented tumor formation and resulted in twitch and tetanic forces similar to those in animals transplanted acutely after denervation.
Interpretation: Despite a neural differentiation protocol, embryonic stem cell-derived motoneurons still carry a risk of tumorigenicity. Pretreating with an antimitotic agent leads to survival and functional muscle reinnervation if performed within 4 weeks of denervation in the mouse.
Figures
References
-
- Bromberg MB. Quality of life in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am 2008;19:591–605. - PubMed
-
- Boakye M, Leigh BC, Skelly AC. Quality of life in persons with spinal cord injury: comparisons with other populations. J Neurosurg Spine 2012;17(1 Suppl):29–37. - PubMed
-
- Carlstedt T, Cullheim S. Spinal cord motoneuron maintenance, injury and repair. Prog Brain Res 2000;127:501–514. - PubMed
-
- Popovic D, Gordon T, Rafuse VF, Prochazka A. Properties of implanted electrodes for functional electrical stimulation. Ann Biomed Eng 1991;19:303–316. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

