LANA-Mediated Recruitment of Host Polycomb Repressive Complexes onto the KSHV Genome during De Novo Infection
- PMID: 27606464
- PMCID: PMC5015872
- DOI: 10.1371/journal.ppat.1005878
LANA-Mediated Recruitment of Host Polycomb Repressive Complexes onto the KSHV Genome during De Novo Infection
Abstract
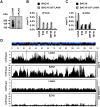
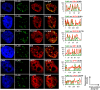
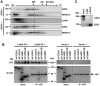
One of the hallmarks of the latent phase of Kaposi's sarcoma-associated herpesvirus (KSHV) infection is the global repression of lytic viral gene expression. Following de novo KSHV infection, the establishment of latency involves the chromatinization of the incoming viral genomes and recruitment of the host Polycomb repressive complexes (PRC1 and PRC2) to the promoters of lytic genes, which is accompanied by the inhibition of lytic genes. However, the mechanism of how PRCs are recruited to the KSHV episome is still unknown. Utilizing a genetic screen of latent genes in the context of KSHV genome, we identified the latency-associated nuclear antigen (LANA) to be responsible for the genome-wide recruitment of PRCs onto the lytic promoters following infection. We found that LANA initially bound to the KSHV genome right after infection and subsequently recruited PRCs onto the viral lytic promoters, thereby repressing lytic gene expression. Furthermore, both the DNA and chromatin binding activities of LANA were required for the binding of LANA to the KSHV promoters, which was necessary for the recruitment of PRC2 to the lytic promoters during de novo KSHV infection. Consequently, the LANA-knockout KSHV could not recruit PRCs to its viral genome upon de novo infection, resulting in aberrant lytic gene expression and dysregulation of expression of host genes involved in cell cycle and proliferation pathways. In this report, we demonstrate that KSHV LANA recruits host PRCs onto the lytic promoters to suppress lytic gene expression following de novo infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
IFI16 recruits HDAC1 and HDAC2 to deacetylate the Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen (LANA), facilitating latency.J Virol. 2025 Mar 18;99(3):e0154924. doi: 10.1128/jvi.01549-24. Epub 2025 Feb 10. J Virol. 2025. PMID: 39927772 Free PMC article.
-
Epigenetic factor siRNA screen during primary KSHV infection identifies novel host restriction factors for the lytic cycle of KSHV.PLoS Pathog. 2020 Jan 10;16(1):e1008268. doi: 10.1371/journal.ppat.1008268. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31923286 Free PMC article.
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
-
An atlas of chromatin landscape in KSHV-infected cells during de novo infection and reactivation.Virology. 2024 Sep;597:110146. doi: 10.1016/j.virol.2024.110146. Epub 2024 Jun 19. Virology. 2024. PMID: 38909515 Review.
Cited by
-
Characterization of de novo lytic infection of dermal lymphatic microvascular endothelial cells by Kaposi's sarcoma-associated herpesvirus.Virology. 2019 Oct;536:27-31. doi: 10.1016/j.virol.2019.07.028. Epub 2019 Jul 31. Virology. 2019. PMID: 31394409 Free PMC article.
-
Epigenetic and epitranscriptomic regulation during oncogenic γ-herpesvirus infection.Front Microbiol. 2025 Jan 7;15:1484455. doi: 10.3389/fmicb.2024.1484455. eCollection 2024. Front Microbiol. 2025. PMID: 39839102 Free PMC article. Review.
-
KSHV RTA utilizes the host E3 ubiquitin ligase complex RNF20/40 to drive lytic reactivation.J Virol. 2023 Nov 30;97(11):e0138923. doi: 10.1128/jvi.01389-23. Epub 2023 Oct 27. J Virol. 2023. PMID: 37888983 Free PMC article.
-
Towards Better Understanding of KSHV Life Cycle: from Transcription and Posttranscriptional Regulations to Pathogenesis.Virol Sin. 2019 Apr;34(2):135-161. doi: 10.1007/s12250-019-00114-3. Epub 2019 Apr 25. Virol Sin. 2019. PMID: 31025296 Free PMC article. Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865–1869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA197153/CA/NCI NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- R01 DE025465/DE/NIDCR NIH HHS/United States
- R03 DE025562/DE/NIDCR NIH HHS/United States
- R01 CA177377/CA/NCI NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R35 CA200422/CA/NCI NIH HHS/United States
- R01 HL110609/HL/NHLBI NIH HHS/United States
- R01 CA096512/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- R01 AI116585/AI/NIAID NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R21 AI119597/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

