Rice Dwarf Virus P2 Protein Hijacks Auxin Signaling by Directly Targeting the Rice OsIAA10 Protein, Enhancing Viral Infection and Disease Development
- PMID: 27606959
- PMCID: PMC5015840
- DOI: 10.1371/journal.ppat.1005847
Rice Dwarf Virus P2 Protein Hijacks Auxin Signaling by Directly Targeting the Rice OsIAA10 Protein, Enhancing Viral Infection and Disease Development
Abstract
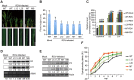
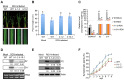
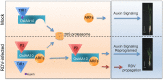
The phytohormone auxin plays critical roles in regulating myriads of plant growth and developmental processes. Microbe infection can disturb auxin signaling resulting in defects in these processes, but the underlying mechanisms are poorly understood. Auxin signaling begins with perception of auxin by a transient co-receptor complex consisting of an F-box transport inhibitor response 1/auxin signaling F-box (TIR1/AFB) protein and an auxin/indole-3-acetic acid (Aux/IAA) protein. Auxin binding to the co-receptor triggers ubiquitination and 26S proteasome degradation of the Aux/IAA proteins, leading to subsequent events, including expression of auxin-responsive genes. Here we report that Rice dwarf virus (RDV), a devastating pathogen of rice, causes disease symptoms including dwarfing, increased tiller number and short crown roots in infected rice as a result of reduced sensitivity to auxin signaling. The RDV capsid protein P2 binds OsIAA10, blocking the interaction between OsIAA10 and OsTIR1 and inhibiting 26S proteasome-mediated OsIAA10 degradation. Transgenic rice plants overexpressing wild-type or a dominant-negative (degradation-resistant) mutant of OsIAA10 phenocopy RDV symptoms are more susceptible to RDV infection; however, knockdown of OsIAA10 enhances the resistance of rice to RDV infection. Our findings reveal a previously unknown mechanism of viral protein reprogramming of a key step in auxin signaling initiation that enhances viral infection and pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus.PLoS Pathog. 2020 Dec 2;16(12):e1009118. doi: 10.1371/journal.ppat.1009118. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33264360 Free PMC article.
-
The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms.Plant Physiol. 2005 Dec;139(4):1935-45. doi: 10.1104/pp.105.072306. Epub 2005 Nov 18. Plant Physiol. 2005. PMID: 16299167 Free PMC article.
-
Control of grain size in rice by TGW3 phosphorylation of OsIAA10 through potentiation of OsIAA10-OsARF4-mediated auxin signaling.Cell Rep. 2023 Mar 28;42(3):112187. doi: 10.1016/j.celrep.2023.112187. Epub 2023 Mar 3. Cell Rep. 2023. PMID: 36871218
-
Auxin receptors and plant development: a new signaling paradigm.Annu Rev Cell Dev Biol. 2008;24:55-80. doi: 10.1146/annurev.cellbio.23.090506.123214. Annu Rev Cell Dev Biol. 2008. PMID: 18631113 Review.
-
26S Proteasome: Hunter and Prey in Auxin Signaling.Trends Plant Sci. 2016 Jul;21(7):546-548. doi: 10.1016/j.tplants.2016.05.007. Epub 2016 May 27. Trends Plant Sci. 2016. PMID: 27246455 Review.
Cited by
-
Functional analysis revealed the involvement of ZmABCB15 in resistance to rice black-streaked dwarf virus infection.BMC Plant Biol. 2022 Oct 11;22(1):484. doi: 10.1186/s12870-022-03861-w. BMC Plant Biol. 2022. PMID: 36217105 Free PMC article.
-
A Review of Vector-Borne Rice Viruses.Viruses. 2022 Oct 14;14(10):2258. doi: 10.3390/v14102258. Viruses. 2022. PMID: 36298813 Free PMC article. Review.
-
BPB1 regulates rice (Oryza sative L.) panicle length and panicle branch development by promoting lignin and inhibiting cellulose accumulation.Mol Breed. 2023 May 6;43(5):41. doi: 10.1007/s11032-023-01389-x. eCollection 2023 May. Mol Breed. 2023. PMID: 37312745 Free PMC article.
-
Current understanding of the interplays between host hormones and plant viral infections.PLoS Pathog. 2021 Feb 25;17(2):e1009242. doi: 10.1371/journal.ppat.1009242. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33630970 Free PMC article. Review.
-
Identification of One Major QTL and a Novel Gene OsIAA17q5 Associated with Tiller Number in Rice Using QTL Analysis.Plants (Basel). 2022 Feb 17;11(4):538. doi: 10.3390/plants11040538. Plants (Basel). 2022. PMID: 35214873 Free PMC article.
References
-
- Soosaar JLM, Burch-Smith TM, Dinesh-Kumar SP (2005) Mechanisms of plant resistance to viruses. Nat Rev Microbiol 3: 789–798. - PubMed
-
- Wang A (2014) Dissecting the molecular network of virus-plant interactions: the complex roles of host factors. Annu Rev Phytopathol 53: 1–22. - PubMed
-
- Jameson PE, Clarke SF (2002) Hormone-virus interactions in plants. CRC Crit Rev Plant Sci 21: 205–228.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

