Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture
- PMID: 27607106
- PMCID: PMC5015985
- DOI: 10.1371/journal.pone.0162508
Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture
Abstract
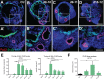
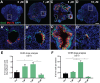
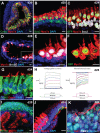
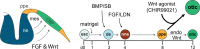
Stem cell-derived inner ear sensory epithelia are a promising source of tissues for treating patients with hearing loss and dizziness. We recently demonstrated how to generate inner ear sensory epithelia, designated as inner ear organoids, from mouse embryonic stem cells (ESCs) in a self-organizing 3D culture. Here we improve the efficiency of this culture system by elucidating how Wnt signaling activity can drive the induction of otic tissue. We found that a carefully timed treatment with the potent Wnt agonist CHIR99021 promotes induction of otic vesicles-a process that was previously self-organized by unknown mechanisms. The resulting otic-like vesicles have a larger lumen size and contain a greater number of Pax8/Pax2-positive otic progenitor cells than organoids derived without the Wnt agonist. Additionally, these otic-like vesicles give rise to large inner ear organoids with hair cells whose morphological, biochemical and functional properties are indistinguishable from those of vestibular hair cells in the postnatal mouse inner ear. We conclude that Wnt signaling plays a similar role during inner ear organoid formation as it does during inner ear development in the embryo.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests. There is a pending international patent application relevant to this study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
From Otic Induction to Hair Cell Production: Pax2EGFP Cell Line Illuminates Key Stages of Development in Mouse Inner Ear Organoid Model.Stem Cells Dev. 2018 Feb 15;27(4):237-251. doi: 10.1089/scd.2017.0142. Epub 2018 Jan 29. Stem Cells Dev. 2018. PMID: 29272992 Free PMC article.
-
Wnt Modulation Enhances Otic Differentiation by Facilitating the Enucleation Process but Develops Unnecessary Cardiac Structures.Int J Mol Sci. 2021 Sep 24;22(19):10306. doi: 10.3390/ijms221910306. Int J Mol Sci. 2021. PMID: 34638648 Free PMC article.
-
Generation of inner ear organoids from human pluripotent stem cells.Methods Cell Biol. 2020;159:303-321. doi: 10.1016/bs.mcb.2020.02.006. Epub 2020 Mar 11. Methods Cell Biol. 2020. PMID: 32586448
-
Patterning and cell fate in the inner ear: a case for Notch in the chicken embryo.Dev Growth Differ. 2013 Jan;55(1):96-112. doi: 10.1111/dgd.12016. Epub 2012 Dec 17. Dev Growth Differ. 2013. PMID: 23252974 Review.
-
Building inner ears: recent advances and future challenges for in vitro organoid systems.Cell Death Differ. 2021 Jan;28(1):24-34. doi: 10.1038/s41418-020-00678-8. Epub 2020 Dec 14. Cell Death Differ. 2021. PMID: 33318601 Free PMC article. Review.
Cited by
-
Grainyhead-like 2 is required for morphological integrity of mouse embryonic stem cells and orderly formation of inner ear-like organoids.Front Cell Dev Biol. 2023 Sep 7;11:1112069. doi: 10.3389/fcell.2023.1112069. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37745294 Free PMC article.
-
Organoids-the key to novel therapies for the inner ear?HNO. 2024 Dec;72(Suppl 2):83-88. doi: 10.1007/s00106-023-01367-x. Epub 2024 May 22. HNO. 2024. PMID: 38775829 Review. English.
-
Protocol for vibratome sectioning, immunofluorescence, and S-phase labeling of inner ear organoids.STAR Protoc. 2025 Aug 14;6(3):104032. doi: 10.1016/j.xpro.2025.104032. Online ahead of print. STAR Protoc. 2025. PMID: 40815565 Free PMC article.
-
Fbxo2VHC mouse and embryonic stem cell reporter lines delineate in vitro-generated inner ear sensory epithelia cells and enable otic lineage selection and Cre-recombination.Dev Biol. 2018 Nov 1;443(1):64-77. doi: 10.1016/j.ydbio.2018.08.013. Epub 2018 Sep 1. Dev Biol. 2018. PMID: 30179592 Free PMC article.
-
Otic Organoids Containing Spiral Ganglion Neuron-like Cells Derived from Human-induced Pluripotent Stem Cells as a Model of Drug-induced Neuropathy.Stem Cells Transl Med. 2022 Mar 31;11(3):282-296. doi: 10.1093/stcltm/szab023. Stem Cells Transl Med. 2022. PMID: 35356976 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

