Alpha1-Antitrypsin Attenuates Renal Fibrosis by Inhibiting TGF-β1-Induced Epithelial Mesenchymal Transition
- PMID: 27607429
- PMCID: PMC5015906
- DOI: 10.1371/journal.pone.0162186
Alpha1-Antitrypsin Attenuates Renal Fibrosis by Inhibiting TGF-β1-Induced Epithelial Mesenchymal Transition
Abstract
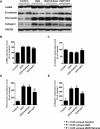
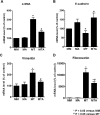
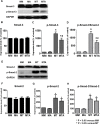
Alpha1-antitrypsin (AAT) exerts its anti-inflammatory effect through regulating the activity of serine proteinases. This study evaluated the inhibitory effects of AAT against the transforming growth factor (TGF)-β1 induced epithelial-to-mesenchymal transition (EMT) in unilateral ureter obstruction (UUO) mice and Madin-Darby canine kidney (MDCK) cells. C57BL/6 mice with induced UUO were injected intraperitoneally with AAT (80 mg/Kg) or vehicle for 7 days. MDCK cells were treated with TGF-β1 (2 ng/mL) for 48 hours to induce EMT, and co-treated with AAT (10 mg/mL) to inhibit the EMT. Masson's trichrome and Sirius red staining was used to estimate the extent of renal fibrosis in UUO mice. The expression of alpha-smooth muscle actin (α-SMA), vimentin, fibronectin, collagen I, and E-cadherin in MDCK cells and kidney tissue were evaluated. Masson's and Sirius red staining revealed that the area of renal fibrosis was significantly smaller in AAT treated UUO group compared with that of UUO and vehicle treated UUO groups. AAT treatment attenuated upregulation of Smad2/3 phosphorylation in UUO mouse model. Co-treatment of MDCK cells with TGF-β1 and AAT significantly attenuated the changes in the expression of α-SMA, vimentin, fibronectin, collagen I, and E-cadherin. AAT also decreased the phosphorylated Smad3 expression and the phosphorylated Smad3/Smad3 ratio in MDCK cells. AAT treatment inhibited EMT induced by TGF-β1 in MDCK cells and attenuated renal fibrosis in the UUO mouse model. The results of this work suggest that AAT could inhibit the process of EMT through the suppression of TGF-β/Smad3 signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

