Feedback Control of Two-Component Regulatory Systems
- PMID: 27607549
- PMCID: PMC8380452
- DOI: 10.1146/annurev-micro-102215-095331
Feedback Control of Two-Component Regulatory Systems
Abstract
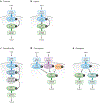
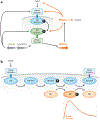
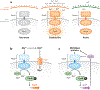
Two-component systems are a dominant form of bacterial signal transduction. The prototypical two-component system consists of a sensor that responds to a specific input(s) by modifying the output of a cognate regulator. Because the output of a two-component system is the amount of phosphorylated regulator, feedback mechanisms may alter the amount of regulator, and/or modify the ability of a sensor or other proteins to alter the phosphorylation state of the regulator. Two-component systems may display intrinsic feedback whereby the amount of phosphorylated regulator changes under constant inducing conditions and without the participation of additional proteins. Feedback control allows a two-component system to achieve particular steady-state levels, to reach a given steady state with distinct dynamics, to express coregulated genes in a given order, and to activate a regulator to different extents, depending on the signal acting on the sensor.
Keywords: expression dynamics; phosphorylation; signal access; transcription surge.
Figures
Similar articles
-
Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor.Genes Dev. 2004 Sep 15;18(18):2302-13. doi: 10.1101/gad.1230804. Genes Dev. 2004. PMID: 15371344 Free PMC article.
-
To ∼P or Not to ∼P? Non-canonical activation by two-component response regulators.Mol Microbiol. 2017 Jan;103(2):203-213. doi: 10.1111/mmi.13532. Epub 2016 Oct 11. Mol Microbiol. 2017. PMID: 27656860 Free PMC article. Review.
-
Response acceleration in post-translationally regulated genetic circuits.J Mol Biol. 2010 Mar 12;396(5):1398-409. doi: 10.1016/j.jmb.2009.11.043. Epub 2009 Nov 20. J Mol Biol. 2010. PMID: 19932119 Free PMC article.
-
General Aspects of Two-Component Regulatory Circuits in Bacteria: Domains, Signals and Roles.Curr Protein Pept Sci. 2017;18(10):990-1004. doi: 10.2174/1389203717666160809154809. Curr Protein Pept Sci. 2017. PMID: 27514854 Review.
-
Temporal and evolutionary dynamics of two-component signaling pathways.Curr Opin Microbiol. 2015 Apr;24:7-14. doi: 10.1016/j.mib.2014.12.003. Epub 2015 Jan 10. Curr Opin Microbiol. 2015. PMID: 25589045 Free PMC article. Review.
Cited by
-
Sensory Systems and Transcriptional Regulation in Escherichia coli.Front Bioeng Biotechnol. 2022 Feb 14;10:823240. doi: 10.3389/fbioe.2022.823240. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35237580 Free PMC article. Review.
-
New Twists and Turns in Bacterial Locomotion and Signal Transduction.J Bacteriol. 2019 Sep 20;201(20):e00439-19. doi: 10.1128/JB.00439-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31358610 Free PMC article. Review.
-
The phosphorelay BarA/SirA activates the non-cognate regulator RcsB in Salmonella enterica.PLoS Genet. 2020 May 11;16(5):e1008722. doi: 10.1371/journal.pgen.1008722. eCollection 2020 May. PLoS Genet. 2020. PMID: 32392214 Free PMC article.
-
Bacterial capsules: Occurrence, mechanism, and function.NPJ Biofilms Microbiomes. 2024 Mar 13;10(1):21. doi: 10.1038/s41522-024-00497-6. NPJ Biofilms Microbiomes. 2024. PMID: 38480745 Free PMC article. Review.
-
Overcoming the Cost of Positive Autoregulation by Accelerating the Response with a Coupled Negative Feedback.Cell Rep. 2018 Sep 11;24(11):3061-3071.e6. doi: 10.1016/j.celrep.2018.08.023. Cell Rep. 2018. PMID: 30208328 Free PMC article.
References
-
- Alloing G, Martin B, Granadel C, Claverys JP. 1998. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol 29:75–83 - PubMed
-
- Balaban NQ. 2011. Persistence: mechanisms for triggering and enhancing phenotypic variability. Curr. Opin. Genet. Dev 21:768–75 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

