Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism
- PMID: 27607575
- PMCID: PMC5059868
- DOI: 10.1038/cddis.2016.265
Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism
Abstract
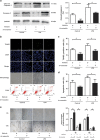
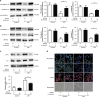
Acute respiratory distress syndrome (ARDS) is characterized by increased pulmonary inflammation and endothelial barrier permeability. Omentin has been shown to benefit obesity-related systemic vascular diseases; however, its effects on ARDS are unknown. In the present study, the level of circulating omentin in patients with ARDS was assessed to appraise its clinical significance in ARDS. Mice were subjected to systemic administration of adenoviral vector expressing omentin (Ad-omentin) and one-shot treatment of recombinant human omentin (rh-omentin) to examine omentin's effects on lipopolysaccharide (LPS)-induced ARDS. Pulmonary endothelial cells (ECs) were treated with rh-omentin to further investigate its underlying mechanism. We found that a decreased level of circulating omentin negatively correlated with white blood cells and procalcitonin in patients with ARDS. Ad-omentin protected against LPS-induced ARDS by alleviating the pulmonary inflammatory response and endothelial barrier injury in mice, accompanied by Akt/eNOS pathway activation. Treatment of pulmonary ECs with rh-omentin attenuated inflammatory response and restored adherens junctions (AJs), and cytoskeleton organization promoted endothelial barrier after LPS insult. Moreover, the omentin-mediated enhancement of EC survival and differentiation was blocked by the Akt/eNOS pathway inactivation. Therapeutic rh-omentin treatment also effectively protected against LPS-induced ARDS via the Akt/eNOS pathway. Collectively, these data indicated that omentin protects against LPS-induced ARDS by suppressing inflammation and promoting the pulmonary endothelial barrier, at least partially, through an Akt/eNOS-dependent mechanism. Therapeutic strategies aiming to restore omentin levels may be valuable for the prevention or treatment of ARDS.
Figures






Similar articles
-
Vaspin protects against LPS‑induced ARDS by inhibiting inflammation, apoptosis and reactive oxygen species generation in pulmonary endothelial cells via the Akt/GSK‑3β pathway.Int J Mol Med. 2017 Dec;40(6):1803-1817. doi: 10.3892/ijmm.2017.3176. Epub 2017 Oct 9. Int J Mol Med. 2017. PMID: 29039444 Free PMC article.
-
Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism.J Biol Chem. 2012 Jan 2;287(1):408-417. doi: 10.1074/jbc.M111.261818. Epub 2011 Nov 11. J Biol Chem. 2012. PMID: 22081609 Free PMC article.
-
Intermedin alleviates the inflammatory response and stabilizes the endothelial barrier in LPS-induced ARDS through the PI3K/Akt/eNOS signaling pathway.Int Immunopharmacol. 2020 Nov;88:106951. doi: 10.1016/j.intimp.2020.106951. Epub 2020 Sep 3. Int Immunopharmacol. 2020. PMID: 32892076
-
Omentin-1: Protective impact on ischemic stroke via ameliorating atherosclerosis.Clin Chim Acta. 2021 Jun;517:31-40. doi: 10.1016/j.cca.2021.02.004. Epub 2021 Feb 16. Clin Chim Acta. 2021. PMID: 33607071 Review.
-
Omentin-A Novel Adipokine in Respiratory Diseases.Int J Mol Sci. 2017 Dec 28;19(1):73. doi: 10.3390/ijms19010073. Int J Mol Sci. 2017. PMID: 29283409 Free PMC article. Review.
Cited by
-
The effects of iron deficient and high iron diets on SARS-CoV-2 lung infection and disease.Front Microbiol. 2024 Sep 4;15:1441495. doi: 10.3389/fmicb.2024.1441495. eCollection 2024. Front Microbiol. 2024. PMID: 39296289 Free PMC article.
-
The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis.Cell Death Dis. 2019 Mar 20;10(4):278. doi: 10.1038/s41419-019-1514-4. Cell Death Dis. 2019. PMID: 30894511 Free PMC article.
-
Anti-inflammatory adipokines: chemerin, vaspin, omentin concentrations and SARS-CoV-2 outcomes.Sci Rep. 2021 Nov 2;11(1):21514. doi: 10.1038/s41598-021-00928-w. Sci Rep. 2021. PMID: 34728695 Free PMC article.
-
Pulmonary vascular dysfunction in metabolic syndrome.J Physiol. 2019 Feb;597(4):1121-1141. doi: 10.1113/JP275856. Epub 2018 Sep 12. J Physiol. 2019. PMID: 30125956 Free PMC article. Review.
-
Fraxin Alleviates LPS-Induced ARDS by Downregulating Inflammatory Responses and Oxidative Damages and Reducing Pulmonary Vascular Permeability.Inflammation. 2019 Oct;42(5):1901-1912. doi: 10.1007/s10753-019-01052-8. Inflammation. 2019. PMID: 31273573
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–2533. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. - PubMed
-
- Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, Kato H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 2004; 28: 237–244. - PubMed
-
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86: 279–367. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

