Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration
- PMID: 27607582
- PMCID: PMC5059849
- DOI: 10.1038/cddis.2016.133
Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration
Abstract
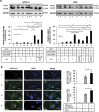
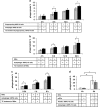
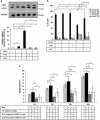
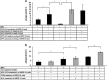
Retinal pigment epithelial (RPE) cells can undergo different forms of cell death, including autophagy-associated cell death during age-related macular degeneration (AMD). Failure of macrophages or dendritic cells (DCs) to engulf the different dying cells in the retina may result in the accumulation of debris and progression of AMD. ARPE-19 and primary human RPE cells undergo autophagy-associated cell death upon serum depletion and oxidative stress induced by hydrogen peroxide (H2O2). Autophagy was revealed by elevated light-chain-3 II (LC3-II) expression and electron microscopy, while autophagic flux was confirmed by blocking the autophago-lysosomal fusion using chloroquine (CQ) in these cells. The autophagy-associated dying RPE cells were engulfed by human macrophages, DCs and living RPE cells in an increasing and time-dependent manner. Inhibition of autophagy by 3-methyladenine (3-MA) decreased the engulfment of the autophagy-associated dying cells by macrophages, whereas sorting out the GFP-LC3-positive/autophagic cell population or treatment by the glucocorticoid triamcinolone (TC) enhanced it. Increased amounts of IL-6 and IL-8 were released when autophagy-associated dying RPEs were engulfed by macrophages. Our data suggest that cells undergoing autophagy-associated cell death engage in clearance mechanisms guided by professional and non-professional phagocytes, which is accompanied by inflammation as part of an in vitro modeling of AMD pathogenesis.
Figures


Similar articles
-
Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation.Int J Mol Sci. 2019 Feb 20;20(4):926. doi: 10.3390/ijms20040926. Int J Mol Sci. 2019. PMID: 30791639 Free PMC article.
-
Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD.Autophagy. 2014;10(11):1989-2005. doi: 10.4161/auto.36184. Autophagy. 2014. PMID: 25484094 Free PMC article.
-
Triamcinolone regulated apopto-phagocytic gene expression patterns in the clearance of dying retinal pigment epithelial cells. A key role of Mertk in the enhanced phagocytosis.Biochim Biophys Acta. 2015 Feb;1850(2):435-46. doi: 10.1016/j.bbagen.2014.10.026. Epub 2014 Oct 27. Biochim Biophys Acta. 2015. PMID: 25450174 Clinical Trial.
-
Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration.Prog Retin Eye Res. 2016 Mar;51:69-89. doi: 10.1016/j.preteyeres.2015.09.002. Epub 2015 Sep 4. Prog Retin Eye Res. 2016. PMID: 26344735 Free PMC article. Review.
-
Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration.Cell Biol Toxicol. 2017 Apr;33(2):113-128. doi: 10.1007/s10565-016-9371-8. Epub 2016 Nov 29. Cell Biol Toxicol. 2017. PMID: 27900566 Free PMC article. Review.
Cited by
-
The Role of Autophagy in Eye Diseases.Life (Basel). 2021 Feb 27;11(3):189. doi: 10.3390/life11030189. Life (Basel). 2021. PMID: 33673657 Free PMC article. Review.
-
Assessment of a Small Molecule Synthetic Lignan in Enhancing Oxidative Balance and Decreasing Lipid Accumulation in Human Retinal Pigment Epithelia.Int J Mol Sci. 2021 May 28;22(11):5764. doi: 10.3390/ijms22115764. Int J Mol Sci. 2021. PMID: 34071220 Free PMC article.
-
Autophagy: a new mechanism for regulating VEGF and PEDF expression in retinal pigment epithelium cells.Int J Ophthalmol. 2019 Apr 18;12(4):557-562. doi: 10.18240/ijo.2019.04.05. eCollection 2019. Int J Ophthalmol. 2019. PMID: 31024806 Free PMC article.
-
Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration.EXCLI J. 2020 Sep 25;19:1353-1371. doi: 10.17179/excli2020-2915. eCollection 2020. EXCLI J. 2020. PMID: 33192217 Free PMC article. Review.
-
Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation.Int J Mol Sci. 2019 Feb 20;20(4):926. doi: 10.3390/ijms20040926. Int J Mol Sci. 2019. PMID: 30791639 Free PMC article.
References
-
- Wada Y, Sun-Wada GH, Kawamura N, Aoyama M. Role of autophagy in embryogenesis. Curr Opin Genet Dev 2014; 27: 60–66. - PubMed
-
- Hu Z, Yang B, Mo X, Xiao H. Mechanism and regulation of autophagy and its role in neuronal diseases. Mol Neurobiol 2014; 52: 1190–1209. - PubMed
-
- Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica 2010; 224 (Suppl 1): 25–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

