Exploratory analysis of ERCC2 DNA methylation in survival among pediatric medulloblastoma patients
- PMID: 27607585
- PMCID: PMC5050141
- DOI: 10.1016/j.canep.2016.08.020
Exploratory analysis of ERCC2 DNA methylation in survival among pediatric medulloblastoma patients
Abstract
Aim: Medulloblastoma is the most frequent malignant pediatric brain tumor. While survival rates have improved due to multimodal treatment including cisplatin-based chemotherapy, there are few prognostic factors for adverse treatment outcomes. Notably, genes involved in the nucleotide excision repair pathway, including ERCC2, have been implicated in cisplatin sensitivity in other cancers. Therefore, this study evaluated the role of ERCC2 DNA methylation profiles on pediatric medulloblastoma survival.
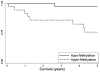
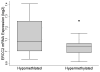
Methods: The study population included 71 medulloblastoma patients (age <18years at diagnosis) and recruited from Texas Children's Cancer Center between 2004 and 2009. DNA methylation profiles were generated from peripheral blood samples using the Illumina Infinium Human Methylation 450 Beadchip. Sixteen ERCC2-associated CpG sites were evaluated in this analysis. Multivariable regression models were used to determine the adjusted association between DNA methylation and survival. Cox regression and Kaplan-Meier curves were used to compare 5-year overall survival between hyper- and hypo-methylation at each CpG site.
Results: In total, 12.7% (n=9) of the patient population died within five years of diagnosis. In our population, methylation of the cg02257300 probe (Hazard Ratio=9.33; 95% Confidence Interval: 1.17-74.64) was associated with death (log-rank p=0.01). This association remained suggestive after correcting for multiple comparisons (FDR p<0.2). No other ERCC2-associated CpG site was associated with survival in this population of pediatric medulloblastoma patients.
Conclusion: These findings provide the first evidence that DNA methylation within the promoter region of the ERCC2 gene may be associated with survival in pediatric medulloblastoma. If confirmed in future studies, this information may lead to improved risk stratification or promote the development of novel, targeted therapeutics.
Keywords: Medulloblastoma; Methylation; Survival.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors report any conflicts of interest.
Figures
Similar articles
-
Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort.Lancet Oncol. 2018 Jun;19(6):785-798. doi: 10.1016/S1470-2045(18)30242-0. Epub 2018 May 9. Lancet Oncol. 2018. PMID: 29753700 Free PMC article.
-
Desmoplastic/nodular medulloblastomas (DNMB) and medulloblastomas with extensive nodularity (MBEN) disclose similar epigenetic signatures but different transcriptional profiles.Acta Neuropathol. 2019 Jun;137(6):1003-1015. doi: 10.1007/s00401-019-01981-6. Epub 2019 Mar 2. Acta Neuropathol. 2019. PMID: 30826918
-
Subgroup and subtype-specific outcomes in adult medulloblastoma.Acta Neuropathol. 2021 Nov;142(5):859-871. doi: 10.1007/s00401-021-02358-4. Epub 2021 Aug 18. Acta Neuropathol. 2021. PMID: 34409497 Free PMC article.
-
New Approaches in Targeted Therapy for Medulloblastoma in Children.Anticancer Res. 2021 Apr;41(4):1715-1726. doi: 10.21873/anticanres.14936. Anticancer Res. 2021. PMID: 33813375 Review.
-
Tumor suppressor genes and medulloblastoma.J Neurooncol. 1996 Jul;29(1):103-12. doi: 10.1007/BF00165523. J Neurooncol. 1996. PMID: 8817421 Review.
Cited by
-
Epigenetic-smoking interaction reveals histologically heterogeneous effects of TRIM27 DNA methylation on overall survival among early-stage NSCLC patients.Mol Oncol. 2020 Nov;14(11):2759-2774. doi: 10.1002/1878-0261.12785. Epub 2020 Sep 3. Mol Oncol. 2020. PMID: 33448640 Free PMC article.
References
-
- Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):83–103. - PubMed
-
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. The Lancet Oncology. 2006;7(10):813–820. - PubMed
-
- Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(11):1400–1407. - PMC - PubMed
-
- Northcott PA, Korshunov A, Pfister SM, et al. The clinical implications of medulloblastoma subgroups. Nature reviews Neurology. 2012;8(6):340–351. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

