Lytic transglycosylases LtgA and LtgD perform distinct roles in remodeling, recycling and releasing peptidoglycan in Neisseria gonorrhoeae
- PMID: 27608412
- PMCID: PMC5463997
- DOI: 10.1111/mmi.13496
Lytic transglycosylases LtgA and LtgD perform distinct roles in remodeling, recycling and releasing peptidoglycan in Neisseria gonorrhoeae
Abstract
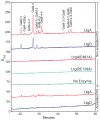
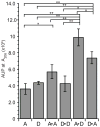
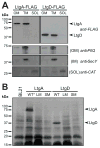
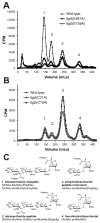
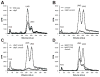
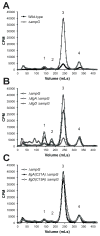
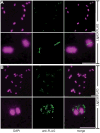
Neisseria gonorrhoeae releases peptidoglycan (PG) fragments during infection that provoke a large inflammatory response and, in pelvic inflammatory disease, this response leads to the death and sloughing of ciliated cells of the Fallopian tube. We characterized the biochemical functions and localization of two enzymes responsible for the release of proinflammatory PG fragments. The putative lytic transglycosylases LtgA and LtgD were shown to create the 1,6-anhydromuramyl moieties, and both enzymes were able to digest a small, synthetic tetrasaccharide dipeptide PG fragment into the cognate 1,6-anhydromuramyl-containing reaction products. Degradation of tetrasaccharide PG fragments by LtgA is the first demonstration of a family 1 lytic transglycosylase exhibiting this activity. Pulse-chase experiments in gonococci demonstrated that LtgA produces a larger amount of PG fragments than LtgD, and a vast majority of these fragments are recycled. In contrast, LtgD was necessary for wild-type levels of PG precursor incorporation and produced fragments predominantly released from the cell. Additionally, super-resolution microscopy established that LtgA localizes to the septum, whereas LtgD is localized around the cell. This investigation suggests a model where LtgD produces PG monomers in such a way that these fragments are released, whereas LtgA creates fragments that are mostly taken into the cytoplasm for recycling.
© 2016 John Wiley & Sons Ltd.
Figures

Similar articles
-
Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers.J Bacteriol. 2008 Sep;190(17):5989-94. doi: 10.1128/JB.00506-08. Epub 2008 Jun 20. J Bacteriol. 2008. PMID: 18567658 Free PMC article.
-
Neisseria gonorrhoeae Lytic Transglycosylases LtgA and LtgD Reduce Host Innate Immune Signaling through TLR2 and NOD2.ACS Infect Dis. 2017 Sep 8;3(9):624-633. doi: 10.1021/acsinfecdis.6b00088. Epub 2017 Jun 21. ACS Infect Dis. 2017. PMID: 28585815 Free PMC article.
-
Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils.Cell Microbiol. 2017 Mar;19(3):10.1111/cmi.12662. doi: 10.1111/cmi.12662. Epub 2016 Nov 3. Cell Microbiol. 2017. PMID: 27597434 Free PMC article.
-
The lytic transglycosylases of Neisseria gonorrhoeae.Microb Drug Resist. 2012 Jun;18(3):271-9. doi: 10.1089/mdr.2012.0001. Epub 2012 Mar 20. Microb Drug Resist. 2012. PMID: 22432703 Free PMC article. Review.
-
The Pathogenic Neisseria Use a Streamlined Set of Peptidoglycan Degradation Proteins for Peptidoglycan Remodeling, Recycling, and Toxic Fragment Release.Front Microbiol. 2019 Jan 31;10:73. doi: 10.3389/fmicb.2019.00073. eCollection 2019. Front Microbiol. 2019. PMID: 30766523 Free PMC article. Review.
Cited by
-
Turnover Chemistry and Structural Characterization of the Cj0843c Lytic Transglycosylase of Campylobacter jejuni.Biochemistry. 2021 Apr 13;60(14):1133-1144. doi: 10.1021/acs.biochem.1c00027. Epub 2021 Mar 22. Biochemistry. 2021. PMID: 33749238 Free PMC article.
-
Transcriptional activation of ompA in Neisseria gonorrhoeae mediated by the XRE family member protein NceR.mBio. 2023 Aug 31;14(4):e0124423. doi: 10.1128/mbio.01244-23. Epub 2023 Jun 30. mBio. 2023. PMID: 37387605 Free PMC article.
-
Mutation of mltG increases peptidoglycan fragment release, cell size, and antibiotic susceptibility in Neisseria gonorrhoeae.J Bacteriol. 2023 Dec 19;205(12):e0027723. doi: 10.1128/jb.00277-23. Epub 2023 Dec 1. J Bacteriol. 2023. PMID: 38038461 Free PMC article.
-
The lytic transglycosylase, LtgG, controls cell morphology and virulence in Burkholderia pseudomallei.Sci Rep. 2019 Jul 30;9(1):11060. doi: 10.1038/s41598-019-47483-z. Sci Rep. 2019. PMID: 31363151 Free PMC article.
-
A Single Dual-Function Enzyme Controls the Production of Inflammatory NOD Agonist Peptidoglycan Fragments by Neisseria gonorrhoeae.mBio. 2017 Oct 17;8(5):e01464-17. doi: 10.1128/mBio.01464-17. mBio. 2017. PMID: 29042497 Free PMC article.
References
-
- Blackburn NT, Clarke AJ. Identification of four families of peptidoglycan lytic transglycosylases. J Mol Evol. 2001;52:78–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

