Electrospun carbon nanofibers reinforced 3D porous carbon polyhedra network derived from metal-organic frameworks for capacitive deionization
- PMID: 27608826
- PMCID: PMC5016738
- DOI: 10.1038/srep32784
Electrospun carbon nanofibers reinforced 3D porous carbon polyhedra network derived from metal-organic frameworks for capacitive deionization
Abstract
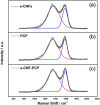
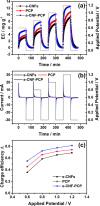
Carbon nanofibers reinforced 3D porous carbon polyhedra network (e-CNF-PCP) was prepared through electrospinning and subsequent thermal treatment. The morphology, structure and electrochemical performance of the e-CNF-PCP were characterized using scanning electron microscopy, Raman spectra, nitrogen adsorption-desorption, cyclic voltammetry and electrochemical impedance spectroscopy, and their electrosorption performance in NaCl solution was studied. The results show that the e-CNF-PCP exhibits a high electrosorption capacity of 16.98 mg g(-1) at 1.2 V in 500 mg l(-1) NaCl solution, which shows great improvement compared with those of electrospun carbon nanofibers and porous carbon polyhedra. The e-CNF-PCP should be a very promising candidate as electrode material for CDI applications.
Figures





Similar articles
-
Fabrication of electrospun trace NiO-doped hierarchical porous carbon nanofiber electrode for capacitive deionization.J Colloid Interface Sci. 2018 Dec 15;532:343-351. doi: 10.1016/j.jcis.2018.07.129. Epub 2018 Jul 30. J Colloid Interface Sci. 2018. PMID: 30096528
-
Facile synthesis of novel graphene sponge for high performance capacitive deionization.Sci Rep. 2015 Feb 13;5:8458. doi: 10.1038/srep08458. Sci Rep. 2015. PMID: 25675835 Free PMC article.
-
Metal-organic framework-derived porous carbon polyhedra for highly efficient capacitive deionization.Chem Commun (Camb). 2015 Aug 4;51(60):12020-3. doi: 10.1039/c5cc03999a. Chem Commun (Camb). 2015. PMID: 26121467
-
Nitrogen-enriched micro-mesoporous carbon derived from polymers organic frameworks for high-performance capacitive deionization.J Environ Sci (China). 2022 Jan;111:282-291. doi: 10.1016/j.jes.2021.03.006. Epub 2021 Apr 16. J Environ Sci (China). 2022. PMID: 34949358
-
Development and Applications of MOFs Derivative One-Dimensional Nanofibers via Electrospinning: A Mini-Review.Nanomaterials (Basel). 2019 Sep 12;9(9):1306. doi: 10.3390/nano9091306. Nanomaterials (Basel). 2019. PMID: 31547339 Free PMC article. Review.
Cited by
-
High-Performance Membrane Capacitive Deionization Based on Metal-Organic Framework-Derived Hierarchical Carbon Structures.ACS Omega. 2018 Aug 1;3(8):8506-8513. doi: 10.1021/acsomega.8b01356. eCollection 2018 Aug 31. ACS Omega. 2018. PMID: 31458979 Free PMC article.
-
Activated Luffa derived biowaste carbon for enhanced desalination performance in brackish water.RSC Adv. 2019 May 14;9(26):14884-14892. doi: 10.1039/c9ra01872g. eCollection 2019 May 9. RSC Adv. 2019. PMID: 35516337 Free PMC article.
-
Layer-by-layer decoration of MOFs on electrospun nanofibers.RSC Adv. 2018 Mar 15;8(19):10509-10515. doi: 10.1039/c8ra01260a. eCollection 2018 Mar 13. RSC Adv. 2018. PMID: 35540460 Free PMC article.
References
-
- Kim S. J., Ko S. H., Kang K. H. & Han J. Direct seawater desalination by ion concentration polarization. Nat Nano 5, 297–301 (2010). - PubMed
-
- Elimelech M. & Phillip W. A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 333, 712–717 (2011). - PubMed
-
- Humplik T. et al.. Nanostructured materials for water desalination. Nanotechnology 22, 292001 (2011). - PubMed
-
- Semiat R. Energy Issues in Desalination Processes. Environ. Sci. Technol. 42, 8193–8201 (2008). - PubMed
-
- Lee J. B., Park K. K., Eum H. M. & Lee C. W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 196, 125–134 (2006).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

