Interaction of MSC with tumor cells
- PMID: 27608835
- PMCID: PMC5016940
- DOI: 10.1186/s12964-016-0143-0
Interaction of MSC with tumor cells
Abstract
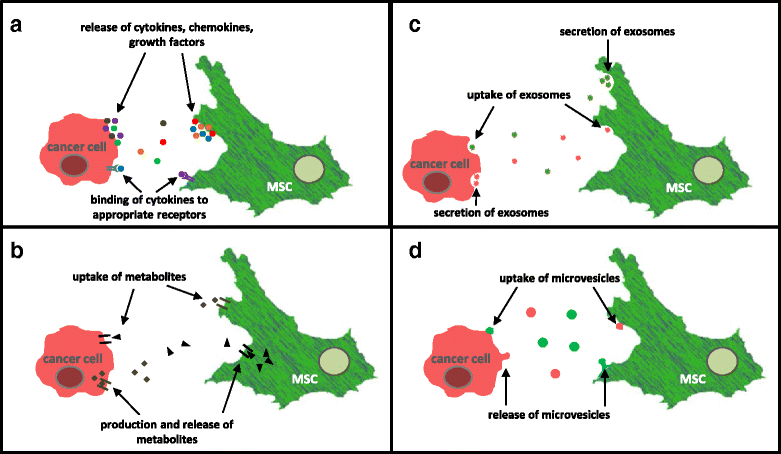
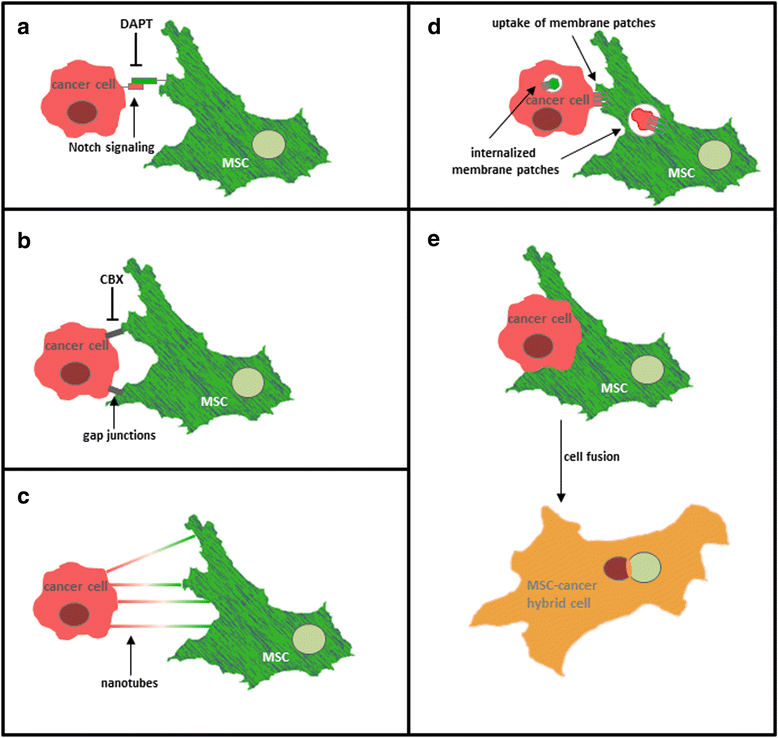
Tumor development and tumor progression is not only determined by the corresponding tumor cells but also by the tumor microenvironment. This includes an orchestrated network of interacting cell types (e.g. immune cells, endothelial cells, fibroblasts, and mesenchymal stroma/stem cells (MSC)) via the extracellular matrix and soluble factors such as cytokines, chemokines, growth factors and various metabolites. Cell populations of the tumor microenvironment can interact directly and indirectly with cancer cells by mutually altering properties and functions of the involved partners. Particularly, mesenchymal stroma/stem cells (MSC) play an important role during carcinogenesis exhibiting different types of intercellular communication. Accordingly, this work focusses on diverse mechanisms of interaction between MSC and cancer cells. Moreover, some functional changes and consequences for both cell types are summarized which can eventually result in the establishment of a carcinoma stem cell niche (CSCN) or the generation of new tumor cell populations by MSC-tumor cell fusion.
Keywords: Cell fusion; Cellular interaction; MSC; Mesenchymal stroma/stem cells; Tumor cell signaling; Tumor microenvironment.
Figures

Similar articles
-
Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-β1.Stem Cells. 2016 Oct;34(10):2536-2547. doi: 10.1002/stem.2412. Epub 2016 Jul 4. Stem Cells. 2016. PMID: 27300750
-
Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment.Int J Cancer. 2016 Jan 1;138(1):14-21. doi: 10.1002/ijc.29417. Epub 2015 Jan 23. Int J Cancer. 2016. PMID: 25559768 Free PMC article. Review.
-
Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells.Mol Cancer. 2017 Feb 1;16(1):28. doi: 10.1186/s12943-017-0595-x. Mol Cancer. 2017. PMID: 28148265 Free PMC article. Review.
-
Multipotent mesenchymal stromal cells promote tumor growth in distinct colorectal cancer cells by a β1-integrin-dependent mechanism.Int J Cancer. 2016 Feb 15;138(4):964-75. doi: 10.1002/ijc.29844. Epub 2015 Oct 1. Int J Cancer. 2016. PMID: 26356035
-
Acquisition of new tumor cell properties by MSC-derived exosomes.Int J Oncol. 2015 Jul;47(1):244-52. doi: 10.3892/ijo.2015.3001. Epub 2015 May 11. Int J Oncol. 2015. PMID: 25963929
Cited by
-
Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration.Int J Nanomedicine. 2019 Jul 29;14:5925-5942. doi: 10.2147/IJN.S217923. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31534331 Free PMC article. Review.
-
Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness.Cancers (Basel). 2020 Dec 11;12(12):3716. doi: 10.3390/cancers12123716. Cancers (Basel). 2020. PMID: 33322354 Free PMC article. Review.
-
Microengineered 3D Tumor Models for Anti-Cancer Drug Discovery in Female-Related Cancers.Ann Biomed Eng. 2021 Aug;49(8):1943-1972. doi: 10.1007/s10439-020-02704-9. Epub 2021 Jan 5. Ann Biomed Eng. 2021. PMID: 33403451 Review.
-
The Intimate Relationship Among EMT, MET and TME: A T(ransdifferentiation) E(nhancing) M(ix) to Be Exploited for Therapeutic Purposes.Cancers (Basel). 2020 Dec 7;12(12):3674. doi: 10.3390/cancers12123674. Cancers (Basel). 2020. PMID: 33297508 Free PMC article. Review.
-
Preventing tumor progression to the bone by induced tumor-suppressing MSCs.Theranostics. 2021 Mar 5;11(11):5143-5159. doi: 10.7150/thno.58779. eCollection 2021. Theranostics. 2021. PMID: 33859739 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

