STA-8666, a novel HSP90 inhibitor/SN-38 drug conjugate, causes complete tumor regression in preclinical mouse models of pediatric sarcoma
- PMID: 27608846
- PMCID: PMC5323173
- DOI: 10.18632/oncotarget.11869
STA-8666, a novel HSP90 inhibitor/SN-38 drug conjugate, causes complete tumor regression in preclinical mouse models of pediatric sarcoma
Abstract
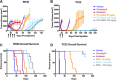
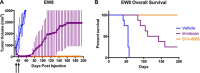
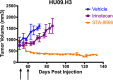
Long-term survival in patients with metastatic, relapsed, or recurrent Ewing sarcoma and rhabdomyosarcoma is dismal. Irinotecan, a topoisomerase 1 inhibitor, has activity in these sarcomas, but due to poor bioavailability of its active metabolite (SN-38) has had limited clinical efficacy. In this study we have evaluated the efficacy and toxicity of STA-8666, a novel drug conjugate which uses an HSP90 inhibitor to facilitate intracellular, tumor-targeted delivery of the topoisomerase 1 inhibitor SN-38, thus preferentially delivering and concentrating SN-38 within tumor tissue. We present in vivo evidence from mouse xenograft models that STA-8666 results in more persistent inhibition of topoisomerase 1 and prolonged DNA damage compared to irinotecan. This translates into superior antitumor efficacy and survival in multiple aggressive models of both diseases in mouse xenografts, as well as in an irinotecan-resistant model of pediatric osteosarcoma, demonstrated by dramatic tumor shrinkage, durable remission and prolonged complete regressions following short-term treatment, compared to conventional irinotecan. Gene expression analysis performed on xenograft tumors treated with either irinotecan or STA-8666 showed that STA-8666 affected expression of DNA damage and repair genes more robustly than irinotecan. These results suggest that STA-8666 may be a promising new agent for patients with pediatric-type sarcoma.
Keywords: SN-38; drug conjugate; pediatric; sarcoma; topoisomerase 1 inhibitor.
Conflict of interest statement
During the bulk of this study, D.A.P was Director of Cancer Biology at Synta Pharmaceuticals. All other authors disclose no potential conflicts of interest.
Figures




Similar articles
-
HSP90 Inhibitor-SN-38 Conjugate Strategy for Targeted Delivery of Topoisomerase I Inhibitor to Tumors.Mol Cancer Ther. 2015 Nov;14(11):2422-32. doi: 10.1158/1535-7163.MCT-15-0455. Epub 2015 Aug 13. Mol Cancer Ther. 2015. PMID: 26271675
-
A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer.Clin Cancer Res. 2016 Oct 15;22(20):5120-5129. doi: 10.1158/1078-0432.CCR-15-3068. Epub 2016 Jun 7. Clin Cancer Res. 2016. PMID: 27267850 Free PMC article.
-
Targeted delivery of chemotherapy using HSP90 inhibitor drug conjugates is highly active against pancreatic cancer models.Oncotarget. 2017 Jan 17;8(3):4399-4409. doi: 10.18632/oncotarget.12642. Oncotarget. 2017. PMID: 27779106 Free PMC article.
-
Irinotecan for pediatric solid tumors: the Memorial Sloan-Kettering experience.J Pediatr Hematol Oncol. 2002 Feb;24(2):101-5. doi: 10.1097/00043426-200202000-00009. J Pediatr Hematol Oncol. 2002. PMID: 11990694 Review.
-
Current Role of Topoisomerase I Inhibitors for the Treatment of Mesenchymal Malignancies and Their Potential Future Use as Payload of Sarcoma-Specific Antibody-Drug Conjugates.Oncol Res Treat. 2024;47(1-2):18-41. doi: 10.1159/000535491. Epub 2023 Nov 28. Oncol Res Treat. 2024. PMID: 38016427 Free PMC article. Review.
Cited by
-
Fusion Oncoproteins in Childhood Cancers: Potential Role in Targeted Therapy.J Pediatr Pharmacol Ther. 2021;26(6):541-555. doi: 10.5863/1551-6776-26.6.541. Epub 2021 Aug 16. J Pediatr Pharmacol Ther. 2021. PMID: 34421403 Free PMC article. Review.
-
Targeting Glycolysis through Inhibition of Lactate Dehydrogenase Impairs Tumor Growth in Preclinical Models of Ewing Sarcoma.Cancer Res. 2019 Oct 1;79(19):5060-5073. doi: 10.1158/0008-5472.CAN-19-0217. Epub 2019 Aug 20. Cancer Res. 2019. PMID: 31431459 Free PMC article.
-
Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) with OT-82 induces DNA damage, cell death, and suppression of tumor growth in preclinical models of Ewing sarcoma.Oncogenesis. 2020 Sep 10;9(9):80. doi: 10.1038/s41389-020-00264-0. Oncogenesis. 2020. PMID: 32908120 Free PMC article.
-
Topoisomerases and cancer chemotherapy: recent advances and unanswered questions.F1000Res. 2019 Sep 30;8:F1000 Faculty Rev-1704. doi: 10.12688/f1000research.20201.1. eCollection 2019. F1000Res. 2019. PMID: 31602296 Free PMC article. Review.
-
Targeting Burkitt lymphoma with a tumor cell-specific heptamethine carbocyanine-cisplatin conjugate.Cancer. 2019 Jul 1;125(13):2222-2232. doi: 10.1002/cncr.32033. Epub 2019 Mar 6. Cancer. 2019. PMID: 30840322 Free PMC article.
References
-
- Dagher R, Helman L. Rhabdomyosarcoma: An overview. Oncologist. 1999;4:34–44. - PubMed
-
- HaDuong JH, Martin AA, Skapek SX, Mascarenhas L. Sarcomas. Pediatr Clin North Am. 2015;62:179–200. - PubMed
-
- Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. - PubMed
-
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

