In vivo bioluminescence imaging for leptomeningeal dissemination of medulloblastoma in mouse models
- PMID: 27609092
- PMCID: PMC5016924
- DOI: 10.1186/s12885-016-2742-y
In vivo bioluminescence imaging for leptomeningeal dissemination of medulloblastoma in mouse models
Abstract
Background: The primary cause of treatment failure in medulloblastomas (MB) is the development of leptomeningeal dissemination (seeding). For translational research on MB seeding, one of the major challenges is the development of reliable experimental models that simulate the seeding and growth characteristics of MBs. To overcome this obstacle, we improved an experimental mouse model by intracisternal inoculation of human MB cells and monitoring with in vivo live images.
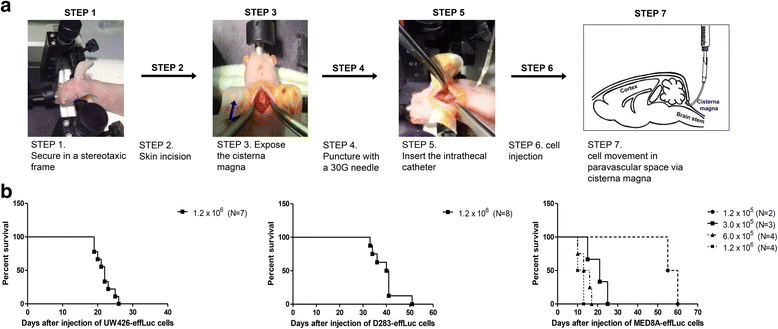
Methods: Human MB cells (UW426, D283 and MED8A) were transfected with a firefly luciferase gene and a Thy1.1 (CD90.1) marker linked with IRES under the control of the CMV promoter in a retroviral DNA backbone (effLuc). The MB-effLuc cells were injected into the cisterna magna using an intrathecal catheter, and bioluminescence images were captured. We performed histopathological analysis to confirm the extent of tumor seeding.
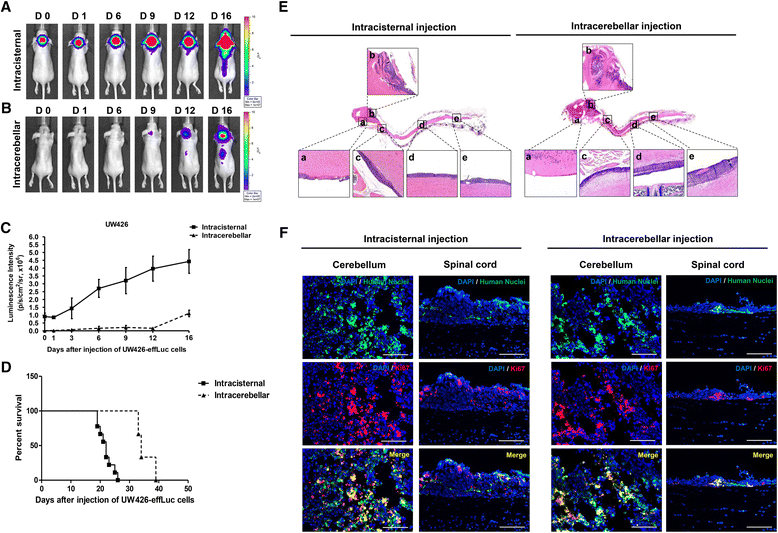
Results: The luciferase activity of MB-effLuc cells displayed a gradually increasing pattern, which correlated with a quantitative luminometric assay. Live imaging showed that the MB-effLuc cells were diffusely distributed in the cervical spinal cord and the lumbosacral area. All mice injected with UW426-effLuc, D283-effLuc and MED8A-effLuc died within 51 days. The median survival was 22, 41 and 12 days after injection of 1.2 × 10(6) UW426-effLuc, D283-effLuc and MED8A-effLuc cells, respectively. The histopathological studies revealed that the MB-effLuc cells spread extensively and diffusely along the leptomeninges of the brain and spinal cord, forming tumor cell-coated layers. The tumor cells in the subarachnoid space expressed a human nuclei marker and Ki-67. Compared with the intracerebellar injection method in which the subfrontal area and distal spinal cord were spared by tumor cell seeding in some mice, the intracisternal injection model more closely resembled the widespread leptomeningeal seeding observed in MB patients.
Conclusion: The results and described method are valuable resources for further translational research to overcome MB seeding.
Keywords: In vivo bioluminescence imaging; Intracisternal injection; Leptomeningeal seeding; Medulloblastoma.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

