Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection
- PMID: 27609178
- PMCID: PMC5016994
- DOI: 10.1186/s12916-016-0680-9
Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection
Abstract
Background: Fecal microbiota transplantation (FMT) has been shown to be safe and effective in treating refractory or relapsing C. difficile infection (CDI), but its use has been limited by practical barriers. We recently reported a small preliminary feasibility study using orally administered frozen fecal capsules. Following these early results, we now report our clinical experience in a large cohort with structured follow-up.
Methods: We prospectively followed a cohort of patients with recurrent or refractory CDI who were treated with frozen, encapsulated FMT at our institution. The primary endpoint was defined as clinical resolution whilst off antibiotics for CDI at 8 weeks after last capsule ingestion. Safety was defined as any FMT-related adverse event grade 2 or above.
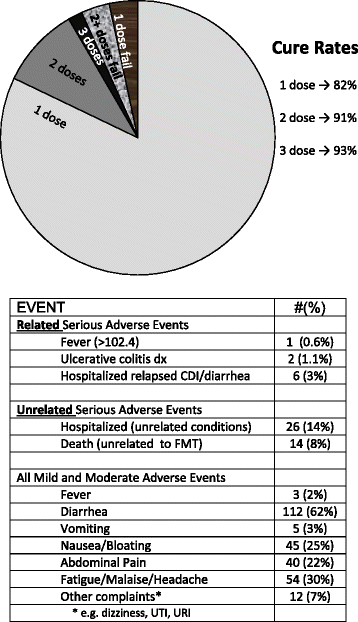
Results: Overall, 180 patients aged 7-95 years with a minimal follow-up of 8 weeks were included in the analysis. CDI resolved in 82 % of patients after a single treatment, rising to a 91 % cure rate with two treatments. Three adverse events Grade 2 or above, deemed related or possibly related to FMT, were observed.
Conclusions: We confirm the effectiveness and safety of oral administration of frozen encapsulated fecal material, prepared from unrelated donors, in treating recurrent CDI. Randomized studies and FMT registries are still needed to ascertain long-term safety.
Keywords: Clostridium difficile; Fecal microbiota transplant; Microbiome; Oral administration.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical