Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: What is missing?
- PMID: 27609196
- PMCID: PMC5140687
- DOI: 10.1016/j.pharmthera.2016.09.009
Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: What is missing?
Abstract
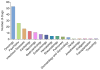
The cardiotoxicity of certain chemotherapeutic agents is now well-established, and has led to the development of the field of cardio-oncology, increased cardiac screening of cancer patients, and limitation of patients' maximum cumulative chemotherapeutic dose. The effect of chemotherapeutic regimes on the heart largely involves cardiomyocyte death, leading to cardiomyopathy and heart failure, or the induction of arrhythmias. Of these cardiotoxic drugs, those resulting in clinical cardiotoxicity can range from 8 to 26% for doxorubicin, 7-28% for trastuzumab, or 5-30% for paclitaxel. For tyrosine kinase inhibitors, QT prolongation and arrhythmia, ischemia and hypertension have been reported in 2-35% of patients. Furthermore, newly introduced chemotherapeutic agents are commonly used as part of changed combinational regimens with significantly increased incidence of cardiotoxicity. It is widely believed that the mechanism of action of these drugs is often independent of their cardiotoxicity, and the basis for why these drugs specifically affect the heart has yet to be established. The genetic rationale for why certain patients experience cardiotoxicity whilst other patients can tolerate high chemotherapy doses has proven highly illusive. This has led to significant genomic efforts using targeted and genome-wide association studies (GWAS) to divine the pharmacogenomic cause of this predilection. With the advent of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), the putative risk and protective role of single nucleotide polymorphisms (SNPs) can now be validated in a human model. Here we review the state of the art knowledge of the genetic predilection to chemotherapy-induced cardiotoxicity and discuss the future for establishing and validating the role of the genome in this disease.
Keywords: Chemotherapy-induced cardiotoxicity; cardiomyopathy; human induced pluripotent stem cells; pharmacogenomics.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


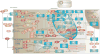

References
-
- Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, Valent P. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. American Journal of Hematology. 2011;86:533–539. - PubMed
-
- Bains OS, Takahashi RH, Pfeifer TA, Grigliatti TA, Reid RE, Riggs KW. Two Allelic Variants of Aldo-Keto Reductase 1A1 Exhibit Reduced in Vitro Metabolism of Daunorubicin. Drug Metabolism and Disposition. 2008;36:904–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

