HIV-1 Tat Induces Unfolded Protein Response and Endoplasmic Reticulum Stress in Astrocytes and Causes Neurotoxicity through Glial Fibrillary Acidic Protein (GFAP) Activation and Aggregation
- PMID: 27609520
- PMCID: PMC5077214
- DOI: 10.1074/jbc.M116.731828
HIV-1 Tat Induces Unfolded Protein Response and Endoplasmic Reticulum Stress in Astrocytes and Causes Neurotoxicity through Glial Fibrillary Acidic Protein (GFAP) Activation and Aggregation
Abstract
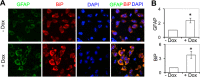
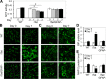
HIV-1 Tat is a major culprit for HIV/neuroAIDS. One of the consistent hallmarks of HIV/neuroAIDS is reactive astrocytes or astrocytosis, characterized by increased cytoplasmic accumulation of the intermediate filament glial fibrillary acidic protein (GFAP). We have shown that that Tat induces GFAP expression in astrocytes and that GFAP activation is indispensable for astrocyte-mediated Tat neurotoxicity. However, the underlying molecular mechanisms are not known. In this study, we showed that Tat expression or GFAP expression led to formation of GFAP aggregates and induction of unfolded protein response (UPR) and endoplasmic reticulum (ER) stress in astrocytes. In addition, we demonstrated that GFAP up-regulation and aggregation in astrocytes were necessary but also sufficient for UPR/ER stress induction in Tat-expressing astrocytes and for astrocyte-mediated Tat neurotoxicity. Importantly, we demonstrated that inhibition of Tat- or GFAP-induced UPR/ER stress by the chemical chaperone 4-phenylbutyrate significantly alleviated astrocyte-mediated Tat neurotoxicity in vitro and in the brain of Tat-expressing mice. Taken together, these results show that HIV-1 Tat expression leads to UPR/ER stress in astrocytes, which in turn contributes to astrocyte-mediated Tat neurotoxicity, and raise the possibility of developing HIV/neuroAIDS therapeutics targeted at UPR/ER stress.
Keywords: Glial fibrillary acidic protein; HIV-1 Tat; astrocytes; endoplasmic reticulum stress; endoplasmic reticulum stress (ER stress); glial cell; human immunodeficiency virus (HIV); neuron; unfolded protein response; unfolded protein response (UPR).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
HIV-1 Tat Promotes Lysosomal Exocytosis in Astrocytes and Contributes to Astrocyte-mediated Tat Neurotoxicity.J Biol Chem. 2016 Oct 21;291(43):22830-22840. doi: 10.1074/jbc.M116.731836. Epub 2016 Sep 8. J Biol Chem. 2016. PMID: 27609518 Free PMC article.
-
Protection against human immunodeficiency virus type 1 Tat neurotoxicity by Ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein.Am J Pathol. 2007 Dec;171(6):1923-35. doi: 10.2353/ajpath.2007.070333. Epub 2007 Nov 30. Am J Pathol. 2007. PMID: 18055541 Free PMC article.
-
Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model.J Neurovirol. 2018 Apr;24(2):168-179. doi: 10.1007/s13365-017-0598-9. Epub 2017 Nov 15. J Neurovirol. 2018. PMID: 29143286 Free PMC article. Review.
-
Involvement of p300 in constitutive and HIV-1 Tat-activated expression of glial fibrillary acidic protein in astrocytes.Glia. 2010 Oct;58(13):1640-8. doi: 10.1002/glia.21038. Glia. 2010. PMID: 20578042 Free PMC article.
-
The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections.Virulence. 2019 Dec;10(1):376-413. doi: 10.1080/21505594.2019.1605803. Virulence. 2019. PMID: 30966844 Free PMC article. Review.
Cited by
-
The Epigenetic Role of miR-124 in HIV-1 Tat- and Cocaine-Mediated Microglial Activation.Int J Mol Sci. 2022 Nov 30;23(23):15017. doi: 10.3390/ijms232315017. Int J Mol Sci. 2022. PMID: 36499350 Free PMC article.
-
4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response.Viruses. 2023 Feb 14;15(2):534. doi: 10.3390/v15020534. Viruses. 2023. PMID: 36851748 Free PMC article.
-
Tat expression led to increased histone 3 tri-methylation at lysine 27 and contributed to HIV latency in astrocytes through regulation of MeCP2 and Ezh2 expression.J Neurovirol. 2019 Aug;25(4):508-519. doi: 10.1007/s13365-019-00751-0. Epub 2019 Apr 24. J Neurovirol. 2019. PMID: 31020497 Free PMC article.
-
HIV-1 infection alters energy metabolism in the brain: Contributions to HIV-associated neurocognitive disorders.Prog Neurobiol. 2019 Oct;181:101616. doi: 10.1016/j.pneurobio.2019.101616. Epub 2019 May 18. Prog Neurobiol. 2019. PMID: 31108127 Free PMC article. Review.
-
HIV-1 and methamphetamine co-treatment in primary human astrocytes: TAARgeting ER/UPR dysfunction.NeuroImmune Pharm Ther. 2024 Feb 19;3(2):139-154. doi: 10.1515/nipt-2023-0020. eCollection 2024 Jun. NeuroImmune Pharm Ther. 2024. PMID: 39175523 Free PMC article.
References
-
- Kaul M., Garden G. A., and Lipton S. A. (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 - PubMed
-
- Kaul M., Zheng J., Okamoto S., Gendelman H. E., and Lipton S. A. (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 12, 878–892 - PubMed
-
- Ellis R. J., Deutsch R., Heaton R. K., Marcotte T. D., McCutchan J. A., Nelson J. A., Abramson I., Thal L. J., Atkinson J. H., Wallace M. R., and Grant I. (1997) Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch. Neurol. 54, 416–424 - PubMed
-
- Ellis R., Langford D., and Masliah E. (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 8, 33–44 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

