Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy
- PMID: 27609653
- PMCID: PMC5337444
- DOI: 10.1016/j.jaci.2016.07.030
Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy
Abstract
Background: Though peanut oral immunotherapy (OIT) is a promising investigational therapy, its potential is limited by substantial adverse events (AEs), which are relatively understudied.
Objective: A retrospective analysis was conducted, pooling data from 3 pediatric peanut OIT trials, comprising the largest analysis of peanut OIT safety to date.
Methods: We pooled data from 104 children with peanut allergy from 3 peanut OIT studies. We catalogued AEs from parental reports, daily symptom diaries, and dose escalations. We included events that were considered likely related to OIT and identified potential baseline predictors of higher AE rates using generalized linear regression models.
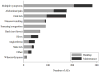
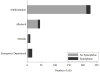
Results: Eighty percent of subjects experienced likely related AEs during OIT (72% during buildup and 47% during maintenance). Of these AEs, over 90% occurred while at home. Approximately 42% of subjects experienced systemic reactions, and 49% experienced gastrointestinal symptoms. Twenty percent of subjects dropped out, with half (10% of the overall group) due to persistent gastrointestinal symptoms. Baseline allergic rhinitis (AR) and peanut SPT wheal size were significant predictors of higher overall AE rates. SPT wheal size predicted increased gastrointestinal AEs, and AR predicted increased systemic reactions. Over the course of OIT, 61% of subjects received treatment for likely related AEs, 59% with antihistamines and 12% with epinephrine.
Conclusions: Peanut OIT is associated with frequent AEs, with rates declining over time, and most graded mild. However, systemic reactions and intolerable gastrointestinal AEs do occur and are significantly associated with AR and peanut SPT wheal size, respectively. Further study is needed of predictive biomarkers and the overall risks and benefits of OIT.
Keywords: Peanut allergy; adverse events; oral immunotherapy; safety.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
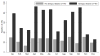

Comment in
-
Recent advances in food allergy prevention and treatment.Ann Allergy Asthma Immunol. 2018 Mar;120(3):241-244. doi: 10.1016/j.anai.2018.01.023. Epub 2018 Feb 2. Ann Allergy Asthma Immunol. 2018. PMID: 29409852 No abstract available.
References
-
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126(6):1105–18. doi: 10.1016/j.jaci.2010.10.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

