Omalizumab facilitates rapid oral desensitization for peanut allergy
- PMID: 27609658
- PMCID: PMC5369605
- DOI: 10.1016/j.jaci.2016.08.010
Omalizumab facilitates rapid oral desensitization for peanut allergy
Abstract
Background: Peanut oral immunotherapy is a promising approach to peanut allergy, but reactions are frequent, and some patients cannot be desensitized. The anti-IgE medication omalizumab (Xolair; Genentech, South San Francisco, Calif) might allow more rapid peanut updosing and decrease reactions.
Objective: We sought to evaluate whether omalizumab facilitated rapid peanut desensitization in highly allergic patients.
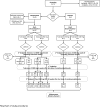
Methods: Thirty-seven subjects were randomized to omalizumab (n = 29) or placebo (n = 8). After 12 weeks of treatment, subjects underwent a rapid 1-day desensitization of up to 250 mg of peanut protein, followed by weekly increases up to 2000 mg. Omalizumab was then discontinued, and subjects continued on 2000 mg of peanut protein. Subjects underwent an open challenge to 4000 mg of peanut protein 12 weeks after stopping study drug. If tolerated, subjects continued on 4000 mg of peanut protein daily.
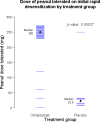
Results: The median peanut dose tolerated on the initial desensitization day was 250 mg for omalizumab-treated subjects versus 22.5 mg for placebo-treated subject. Subsequently, 23 (79%) of 29 subjects randomized to omalizumab tolerated 2000 mg of peanut protein 6 weeks after stopping omalizumab versus 1 (12%) of 8 receiving placebo (P < .01). Twenty-three subjects receiving omalizumab versus 1 subject receiving placebo passed the 4000-mg food challenge. Overall reaction rates were not significantly lower in omalizumab-treated versus placebo-treated subjects (odds ratio, 0.57; P = .15), although omalizumab-treated subjects were exposed to much higher peanut doses.
Conclusion: Omalizumab allows subjects with peanut allergy to be rapidly desensitized over as little as 8 weeks of peanut oral immunotherapy. In the majority of subjects, this desensitization is sustained after omalizumab is discontinued. Additional studies will help clarify which patients would benefit most from this approach.
Keywords: Peanut allergy; desensitization; food allergy; omalizumab.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: DTU is an employee of Genentech; he was affiliated with Boston Children’s Hospital and Harvard Medical School but moved to Genentech after study enrollment began.
Figures

Comment in
-
Recent advances in food allergy prevention and treatment.Ann Allergy Asthma Immunol. 2018 Mar;120(3):241-244. doi: 10.1016/j.anai.2018.01.023. Epub 2018 Feb 2. Ann Allergy Asthma Immunol. 2018. PMID: 29409852 No abstract available.
References
-
- Simons FE. Anaphylaxis: Recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124:625–36. quiz 37–8. - PubMed
-
- Bird JA, Lack G, Perry TT. Clinical management of food allergy. J Allergy Clin Immunol Pract. 2015;3:1–11. quiz 2. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. quiz 8. - PubMed
-
- Lieberman JA, Sicherer SH. Quality of life in food allergy. Curr Opin Allergy Clin Immunol. 2011;11:236–42. - PubMed

