Rethinking Guard Cell Metabolism
- PMID: 27609861
- PMCID: PMC5100799
- DOI: 10.1104/pp.16.00767
Rethinking Guard Cell Metabolism
Abstract
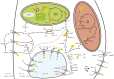
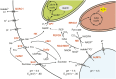
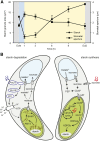
Stomata control gaseous fluxes between the internal leaf air spaces and the external atmosphere and, therefore, play a pivotal role in regulating CO2 uptake for photosynthesis as well as water loss through transpiration. Guard cells, which flank the stomata, undergo adjustments in volume, resulting in changes in pore aperture. Stomatal opening is mediated by the complex regulation of ion transport and solute biosynthesis. Ion transport is exceptionally well understood, whereas our knowledge of guard cell metabolism remains limited, despite several decades of research. In this review, we evaluate the current literature on metabolism in guard cells, particularly the roles of starch, sucrose, and malate. We explore the possible origins of sucrose, including guard cell photosynthesis, and discuss new evidence that points to multiple processes and plasticity in guard cell metabolism that enable these cells to function effectively to maintain optimal stomatal aperture. We also discuss the new tools, techniques, and approaches available for further exploring and potentially manipulating guard cell metabolism to improve plant water use and productivity.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures

Similar articles
-
Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour.New Phytol. 2014 Sep;203(4):1064-1081. doi: 10.1111/nph.12945. New Phytol. 2014. PMID: 25077787 Review.
-
Roles of sucrose in guard cell regulation.New Phytol. 2016 Aug;211(3):809-18. doi: 10.1111/nph.13950. Epub 2016 Apr 6. New Phytol. 2016. PMID: 27060199 Review.
-
Metabolism within the specialized guard cells of plants.New Phytol. 2017 Dec;216(4):1018-1033. doi: 10.1111/nph.14823. Epub 2017 Oct 6. New Phytol. 2017. PMID: 28984366 Review.
-
Toward multifaceted roles of sucrose in the regulation of stomatal movement.Plant Signal Behav. 2018;13(8):e1494468. doi: 10.1080/15592324.2018.1494468. Epub 2018 Aug 1. Plant Signal Behav. 2018. PMID: 30067434 Free PMC article.
-
Guard cell-specific upregulation of sucrose synthase 3 reveals that the role of sucrose in stomatal function is primarily energetic.New Phytol. 2016 Mar;209(4):1470-83. doi: 10.1111/nph.13704. Epub 2015 Oct 15. New Phytol. 2016. PMID: 26467445
Cited by
-
Association mapping and genetic dissection of drought-induced canopy temperature differences in rice.J Exp Bot. 2020 Feb 19;71(4):1614-1627. doi: 10.1093/jxb/erz527. J Exp Bot. 2020. PMID: 31846000 Free PMC article.
-
Transitory Starch Metabolism in Guard Cells: Unique Features for a Unique Function.Plant Physiol. 2017 Jun;174(2):539-549. doi: 10.1104/pp.17.00211. Epub 2017 Mar 14. Plant Physiol. 2017. PMID: 28292855 Free PMC article. Review.
-
Metabolic evidence for distinct pyruvate pools inside plant mitochondria.Nat Plants. 2022 Jun;8(6):694-705. doi: 10.1038/s41477-022-01165-3. Epub 2022 Jun 9. Nat Plants. 2022. PMID: 35681019
-
Guard Cell Starch Degradation Yields Glucose for Rapid Stomatal Opening in Arabidopsis.Plant Cell. 2020 Jul;32(7):2325-2344. doi: 10.1105/tpc.18.00802. Epub 2020 Apr 30. Plant Cell. 2020. PMID: 32354788 Free PMC article.
-
Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth.EMBO Rep. 2020 Aug 5;21(8):e49719. doi: 10.15252/embr.201949719. Epub 2020 Jul 6. EMBO Rep. 2020. PMID: 32627357 Free PMC article.
References
-
- Allaway WG. (1973) Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110: 63–70 - PubMed
-
- Amodeo G, Talbott LD, Zeiger E (1996) Use of potassium and sucrose by onion guard cells during a daily cycle of osmoregulation. Plant Cell Physiol 37: 575–579
-
- Antunes WC, Provart NJ, Williams TCR, Loureiro ME (2012) Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ 35: 747–759 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

