Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers
- PMID: 27609895
- PMCID: PMC5245791
- DOI: 10.1126/science.aaf5171
Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers
Abstract
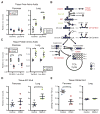
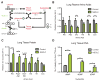
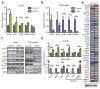
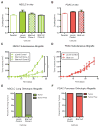
Tumor genetics guides patient selection for many new therapies, and cell culture studies have demonstrated that specific mutations can promote metabolic phenotypes. However, whether tissue context defines cancer dependence on specific metabolic pathways is unknown. Kras activation and Trp53 deletion in the pancreas or the lung result in pancreatic ductal adenocarinoma (PDAC) or non-small cell lung carcinoma (NSCLC), respectively, but despite the same initiating events, these tumors use branched-chain amino acids (BCAAs) differently. NSCLC tumors incorporate free BCAAs into tissue protein and use BCAAs as a nitrogen source, whereas PDAC tumors have decreased BCAA uptake. These differences are reflected in expression levels of BCAA catabolic enzymes in both mice and humans. Loss of Bcat1 and Bcat2, the enzymes responsible for BCAA use, impairs NSCLC tumor formation, but these enzymes are not required for PDAC tumor formation, arguing that tissue of origin is an important determinant of how cancers satisfy their metabolic requirements.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Comment in
-
Location, location, location.Science. 2016 Sep 9;353(6304):1095-6. doi: 10.1126/science.aai7629. Science. 2016. PMID: 27609873 No abstract available.
-
Targeting cancer with tumor-specific therapeutic strategies-metabolic reprogramming beyond the Warburg effect.Transl Cancer Res. 2017 May;6(Suppl 3):S585-S586. doi: 10.21037/tcr.2017.05.13. Transl Cancer Res. 2017. PMID: 28868241 Free PMC article. No abstract available.
-
Branched chain amino acid metabolism and cancer: the importance of keeping things in context.Transl Cancer Res. 2017 May;6(Suppl 3):S578-S584. doi: 10.21037/tcr.2017.05.05. Transl Cancer Res. 2017. PMID: 30613481 Free PMC article. No abstract available.
References
-
- Garraway LA. Genomics-driven oncology: framework for an emerging paradigm. J Clin Oncol. 2013;31:1806–1814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

