TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation
- PMID: 27610006
- PMCID: PMC5005604
- DOI: 10.1155/2016/8319283
TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation
Abstract
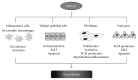
Renal fibrosis, irrespective of its etiology, is a final common stage of almost all chronic kidney diseases. Increased apoptosis, epithelial-to-mesenchymal transition, and inflammatory cell infiltration characterize the injured kidney. On the molecular level, transforming growth factor-β1 (TGF-β1)-Smad3 signaling pathway plays a central role in fibrotic kidney disease. Recent findings indicate the prominent role of microRNAs, small noncoding RNA molecules that inhibit gene expression through the posttranscriptional repression of their target mRNAs, in different pathologic conditions, including renal pathophysiology. miR-21 was also shown to play a dynamic role in inflammatory responses and in accelerating injury responses to promote organ failure and fibrosis. Understanding the cellular and molecular bases of miR-21 involvement in the pathogenesis of kidney diseases, including inflammatory reaction, could be crucial for their early diagnosis. Moreover, the possibility of influencing miR-21 level by specific antagomirs may be considered as an approach for treatment of renal diseases.
Figures


Similar articles
-
TGF-β/Smad signaling in kidney disease.Semin Nephrol. 2012 May;32(3):236-43. doi: 10.1016/j.semnephrol.2012.04.002. Semin Nephrol. 2012. PMID: 22835454 Review.
-
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation.Int J Biol Sci. 2011;7(7):1056-67. doi: 10.7150/ijbs.7.1056. Epub 2011 Sep 2. Int J Biol Sci. 2011. PMID: 21927575 Free PMC article. Review.
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
-
Total Flavonoids from Leaves of Carya Cathayensis Ameliorate Renal Fibrosis via the miR-21/Smad7 Signaling Pathway.Cell Physiol Biochem. 2018;49(4):1551-1563. doi: 10.1159/000493458. Epub 2018 Sep 13. Cell Physiol Biochem. 2018. PMID: 30212825
-
Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway.Nephrol Dial Transplant. 2014 Nov;29(11):2043-53. doi: 10.1093/ndt/gfu240. Epub 2014 Jul 16. Nephrol Dial Transplant. 2014. PMID: 25031017
Cited by
-
Schwann cells-derived exosomal miR-21 participates in high glucose regulation of neurite outgrowth.iScience. 2022 Sep 15;25(10):105141. doi: 10.1016/j.isci.2022.105141. eCollection 2022 Oct 21. iScience. 2022. PMID: 36204278 Free PMC article.
-
Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance.Cells. 2023 Dec 27;13(1):56. doi: 10.3390/cells13010056. Cells. 2023. PMID: 38201260 Free PMC article.
-
Up-regulation of the human-specific CHRFAM7A gene protects against renal fibrosis in mice with obstructive nephropathy.J Cell Mol Med. 2023 Jan;27(1):52-65. doi: 10.1111/jcmm.17630. Epub 2022 Dec 7. J Cell Mol Med. 2023. PMID: 36479618 Free PMC article.
-
Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice.Drug Des Devel Ther. 2018 Aug 6;12:2431-2442. doi: 10.2147/DDDT.S170840. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30122901 Free PMC article.
-
Identification of histone deacetylase 8 as a novel therapeutic target for renal fibrosis.FASEB J. 2020 Jun;34(6):7295-7310. doi: 10.1096/fj.201903254R. Epub 2020 Apr 12. FASEB J. 2020. PMID: 32281211 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

