Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review
- PMID: 27610086
- PMCID: PMC4997013
- DOI: 10.3389/fphys.2016.00361
Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review
Abstract
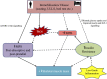
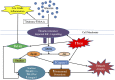
The ever increasing burden of an aging population and pandemic of metabolic syndrome worldwide demands further understanding of the modifiable risk factors in reducing disability and morbidity associated with these conditions. Disuse skeletal muscle atrophy (sometimes referred to as "simple" atrophy) and insulin resistance are "non-pathological" events resulting from sedentary behavior and periods of enforced immobilization e.g., due to fractures or elective orthopedic surgery. Yet, the processes and drivers regulating disuse atrophy and insulin resistance and the associated molecular events remain unclear-especially in humans. The aim of this review is to present current knowledge of relationships between muscle protein turnover, insulin resistance and muscle atrophy during disuse, principally in humans. Immobilization lowers fasted state muscle protein synthesis (MPS) and induces fed-state "anabolic resistance." While a lack of dynamic measurements of muscle protein breakdown (MPB) precludes defining a definitive role for MPB in disuse atrophy, some proteolytic "marker" studies (e.g., MPB genes) suggest a potential early elevation. Immobilization also induces muscle insulin resistance (IR). Moreover, the trajectory of muscle atrophy appears to be accelerated in persistent IR states (e.g., Type II diabetes), suggesting IR may contribute to muscle disuse atrophy under these conditions. Nonetheless, the role of differences in insulin sensitivity across distinct muscle groups and its effects on rates of atrophy remains unclear. Multifaceted time-course studies into the collective role of insulin resistance and muscle protein turnover in the setting of disuse muscle atrophy, in humans, are needed to facilitate the development of appropriate countermeasures and efficacious rehabilitation protocols.
Keywords: diabetes; disuse; immobilization; protein metabolism; skeletal muscle.
Figures
References
-
- Atherton P. J., Paul L. G., Stuart M. P., Sue C. B., Christopher M. A., Charles H. L. (2016). Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab.. [Epub ahead of print]. 10.1152/ajpendo.00257.2016 - DOI - PMC - PubMed
-
- Bamman M. M., Clarke M. S., Feeback D. L., Talmadge R. J., Stevens B. R., Lieberman S. A., et al. . (1998). Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J. Appl. Physiol. (1985) 84, 157–163. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

