Photosynthate Regulation of the Root System Architecture Mediated by the Heterotrimeric G Protein Complex in Arabidopsis
- PMID: 27610112
- PMCID: PMC4997095
- DOI: 10.3389/fpls.2016.01255
Photosynthate Regulation of the Root System Architecture Mediated by the Heterotrimeric G Protein Complex in Arabidopsis
Abstract
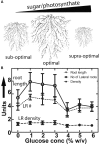
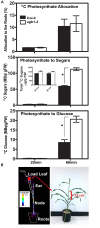
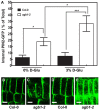
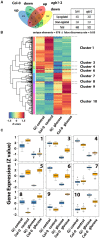
Assimilate partitioning to the root system is a desirable developmental trait to control but little is known of the signaling pathway underlying partitioning. A null mutation in the gene encoding the Gβ subunit of the heterotrimeric G protein complex, a nexus for a variety of signaling pathways, confers altered sugar partitioning in roots. While fixed carbon rapidly reached the roots of wild type and agb1-2 mutant seedlings, agb1 roots had more of this fixed carbon in the form of glucose, fructose, and sucrose which manifested as a higher lateral root density. Upon glucose treatment, the agb1-2 mutant had abnormal gene expression in the root tip validated by transcriptome analysis. In addition, PIN2 membrane localization was altered in the agb1-2 mutant. The heterotrimeric G protein complex integrates photosynthesis-derived sugar signaling incorporating both membrane-and transcriptional-based mechanisms. The time constants for these signaling mechanisms are in the same range as photosynthate delivery to the root, raising the possibility that root cells are able to use changes in carbon fixation in real time to adjust growth behavior.
Keywords: AGB1; PIN2-GFP; gene expression; glucose; lateral root density; photosynthetic partitioning; positron electron tomography imaging.
Figures



Similar articles
-
The heterotrimeric G-protein β subunit, AGB1, plays multiple roles in the Arabidopsis salinity response.Plant Cell Environ. 2015 Oct;38(10):2143-56. doi: 10.1111/pce.12542. Epub 2015 May 12. Plant Cell Environ. 2015. PMID: 25808946
-
The Arabidopsis heterotrimeric G-protein β subunit, AGB1, is required for guard cell calcium sensing and calcium-induced calcium release.Plant J. 2019 Jul;99(2):231-244. doi: 10.1111/tpj.14318. Epub 2019 Apr 30. Plant J. 2019. PMID: 30882980
-
Regulation of root-wave response by extra large and conventional G proteins in Arabidopsis thaliana.Plant J. 2008 Jul;55(2):311-22. doi: 10.1111/j.1365-313X.2008.03506.x. Epub 2008 Apr 4. Plant J. 2008. PMID: 18397373
-
Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi.Mol Plant. 2012 Jan;5(1):98-114. doi: 10.1093/mp/ssr082. Epub 2011 Oct 6. Mol Plant. 2012. PMID: 21980142
-
Plant Morphology of Heterotrimeric G Protein Mutants.Plant Cell Physiol. 2016 Mar;57(3):437-45. doi: 10.1093/pcp/pcw002. Epub 2016 Jan 10. Plant Cell Physiol. 2016. PMID: 26755691 Free PMC article. Review.
Cited by
-
Sweet Immunity: Inulin Boosts Resistance of Lettuce (Lactuca sativa) against Grey Mold (Botrytis cinerea) in an Ethylene-Dependent Manner.Int J Mol Sci. 2019 Feb 28;20(5):1052. doi: 10.3390/ijms20051052. Int J Mol Sci. 2019. PMID: 30823420 Free PMC article.
-
Nitrate-responsive transcriptome analysis of rice RGA1 mutant reveals the role of G-protein alpha subunit in negative regulation of nitrogen-sensitivity and use efficiency.Plant Cell Rep. 2023 Dec;42(12):1987-2010. doi: 10.1007/s00299-023-03078-7. Epub 2023 Oct 24. Plant Cell Rep. 2023. PMID: 37874341
-
Differential regulation of G protein signaling in Arabidopsis through two distinct pathways that internalize AtRGS1.Sci Signal. 2021 Aug 10;14(695):eabe4090. doi: 10.1126/scisignal.abe4090. Sci Signal. 2021. PMID: 34376571 Free PMC article.
-
Crosstalk between heterotrimeric G protein-coupled signaling pathways and WRKY transcription factors modulating plant responses to suboptimal micronutrient conditions.J Exp Bot. 2020 May 30;71(10):3227-3239. doi: 10.1093/jxb/eraa108. J Exp Bot. 2020. PMID: 32107545 Free PMC article.
-
Interplay between ARABIDOPSIS Gβ and WRKY transcription factors differentiates environmental stress responses.Plant Physiol. 2022 Aug 29;190(1):813-827. doi: 10.1093/plphys/kiac305. Plant Physiol. 2022. PMID: 35748759 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

