Fibronectin and Cyclic Strain Improve Cardiac Progenitor Cell Regenerative Potential In Vitro
- PMID: 27610140
- PMCID: PMC5004015
- DOI: 10.1155/2016/8364382
Fibronectin and Cyclic Strain Improve Cardiac Progenitor Cell Regenerative Potential In Vitro
Abstract
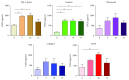
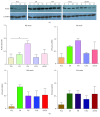
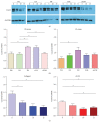
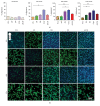
Cardiac progenitor cells (CPCs) have rapidly advanced to clinical trials, yet little is known regarding their interaction with the microenvironment. Signaling cues present in the microenvironment change with development and disease. This work aims to assess the influence of two distinct signaling moieties on CPCs: cyclic biaxial strain and extracellular matrix. We evaluate four endpoints for improving CPC therapy: paracrine signaling, proliferation, connexin43 expression, and alignment. Vascular endothelial growth factor A (about 900 pg/mL) was secreted by CPCs cultured on fibronectin and collagen I. The application of mechanical strain increased vascular endothelial growth factor A secretion 2-4-fold for CPCs cultured on poly-L-lysine, laminin, or a naturally derived cardiac extracellular matrix. CPC proliferation was at least 25% higher on fibronectin than that on other matrices, especially for lower strain magnitudes. At 5% strain, connexin43 expression was highest on fibronectin. With increasing strain magnitude, connexin43 expression decreased by as much as 60% in CPCs cultured on collagen I and a naturally derived cardiac extracellular matrix. Cyclic mechanical strain induced the strongest CPC alignment when cultured on fibronectin or collagen I. This study demonstrates that culturing CPCs on fibronectin with 5% strain magnitude is optimal for their vascular endothelial growth factor A secretion, proliferation, connexin43 expression, and alignment.
Figures


Similar articles
-
Cardiac Progenitor Cells and the Interplay with Their Microenvironment.Stem Cells Int. 2017;2017:7471582. doi: 10.1155/2017/7471582. Epub 2017 Sep 17. Stem Cells Int. 2017. PMID: 29075298 Free PMC article. Review.
-
Construction of vascularized pacemaker tissues by seeding cardiac progenitor cells and endothelial progenitor cells into Matrigel.Life Sci. 2017 Jun 15;179:139-146. doi: 10.1016/j.lfs.2017.05.007. Epub 2017 May 6. Life Sci. 2017. PMID: 28483438
-
Induction of Endothelial Differentiation in Cardiac Progenitor Cells Under Low Serum Conditions.J Vis Exp. 2019 Jan 7;(143). doi: 10.3791/58370. J Vis Exp. 2019. PMID: 30663645
-
Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction.Stem Cells Transl Med. 2016 Aug;5(8):1067-78. doi: 10.5966/sctm.2015-0281. Epub 2016 Jun 22. Stem Cells Transl Med. 2016. PMID: 27334489 Free PMC article.
-
Endothelial and cardiac progenitor cells for cardiovascular repair: A controversial paradigm in cell therapy.Pharmacol Ther. 2018 Jan;181:156-168. doi: 10.1016/j.pharmthera.2017.08.004. Epub 2017 Aug 19. Pharmacol Ther. 2018. PMID: 28827151 Review.
Cited by
-
In vivo evaluation of bioprinted cardiac patches composed of cardiac-specific extracellular matrix and progenitor cells in a model of pediatric heart failure.Biomater Sci. 2022 Jan 18;10(2):444-456. doi: 10.1039/d1bm01539g. Biomater Sci. 2022. PMID: 34878443 Free PMC article.
-
Establishment of Novel Limbus-Derived, Highly Proliferative ABCG2+/ABCB5+ Limbal Epithelial Stem Cell Cultures.Stem Cells Int. 2017;2017:7678637. doi: 10.1155/2017/7678637. Epub 2017 Nov 5. Stem Cells Int. 2017. PMID: 29230251 Free PMC article.
-
A comparative study of the influence of two types of PHEMA stents on the differentiation of ASCs to myocardial cells.Mol Med Rep. 2017 Jul;16(1):507-514. doi: 10.3892/mmr.2017.6680. Epub 2017 Jun 1. Mol Med Rep. 2017. PMID: 28586071 Free PMC article.
-
Cardiac Progenitor Cells and the Interplay with Their Microenvironment.Stem Cells Int. 2017;2017:7471582. doi: 10.1155/2017/7471582. Epub 2017 Sep 17. Stem Cells Int. 2017. PMID: 29075298 Free PMC article. Review.
-
The Vascular Niche for Adult Cardiac Progenitor Cells.Antioxidants (Basel). 2022 Apr 29;11(5):882. doi: 10.3390/antiox11050882. Antioxidants (Basel). 2022. PMID: 35624750 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

