Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents
- PMID: 27610458
- PMCID: PMC5152556
- DOI: 10.1080/13543776.2016.1231805
Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents
Abstract
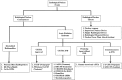
The global threat of a chemical, biological, radiological, or nuclear (CBRN) disaster is an important priority for all government agencies involved in domestic security and public health preparedness. Radiological/nuclear (RN) attacks or accidents have become a larger focus of the United States Food and Drug administration (US FDA) over time because of their increased likeliness. Clinical signs and symptoms of a developing acute radiation syndrome (ARS) are grouped into three sub-syndromes named for the dominant organ system affected, namely the hematopoietic (H-ARS), gastrointestinal (GI-ARS), and neurovascular systems. The availability of safe and effective countermeasures against radiological/nuclear threats currently represents a significant unmet medical need. Areas covered: This article reviews the development of RN threat medical countermeasures and highlights those specific countermeasures that have been recently patented and approved following the FDA Animal Rule. Patents for such agents from 2015 have been presented. Expert opinion: Two granulocyte colony-stimulating factor (G-CSF)-based radiation countermeasures (Neupogen® (Amgen, Thousand Oaks, CA) and Neulasta® (Amgen, Thousand Oaks, CA)) have recently been approved by the FDA for treatment of H-ARS and both these agents are radiomitigators, used after radiation exposure. To date, there are no FDA-approved radioprotectors for ARS.
Keywords: Animal Rule; countermeasures; food and drug administration; radiological and nuclear threats.
Figures
References
-
- Bushberg JT, Kroger LA, Hartman MB. Nuclear/radiological terrorism: emergency department management of radiation casualties. J Emerg Med. 2007;32:71–85. - PubMed
-
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
-
- Flynn DF, Goans RE. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am. 2006;86:601–636. - PubMed
-
- Stone HB, Coleman CN, Anscher MS. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
