Preovulatory Aging In Vivo and In Vitro Affects Maturation Rates, Abundance of Selected Proteins, Histone Methylation Pattern and Spindle Integrity in Murine Oocytes
- PMID: 27611906
- PMCID: PMC5017692
- DOI: 10.1371/journal.pone.0162722
Preovulatory Aging In Vivo and In Vitro Affects Maturation Rates, Abundance of Selected Proteins, Histone Methylation Pattern and Spindle Integrity in Murine Oocytes
Abstract
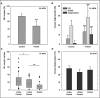
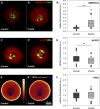
Delayed ovulation and delayed fertilization can lead to reduced developmental competence of the oocyte. In contrast to the consequences of postovulatory aging of the oocyte, hardly anything is known about the molecular processes occurring during oocyte maturation if ovulation is delayed (preovulatory aging). We investigated several aspects of oocyte maturation in two models of preovulatory aging: an in vitro follicle culture and an in vivo mouse model in which ovulation was postponed using the GnRH antagonist cetrorelix. Both models showed significantly reduced oocyte maturation rates after aging. Furthermore, in vitro preovulatory aging deregulated the protein abundance of the maternal effect genes Smarca4 and Nlrp5, decreased the levels of histone H3K9 trimethylation and caused major deterioration of chromosome alignment and spindle conformation. Protein abundance of YBX2, an important regulator of mRNA stability, storage and recruitment in the oocyte, was not affected by in vitro aging. In contrast, in vivo preovulatory aging led to reduction in Ybx2 transcript and YBX2 protein abundance. Taken together, preovulatory aging seems to affect various processes in the oocyte, which could explain the low maturation rates and the previously described failures in fertilization and embryonic development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes.Hum Reprod. 2016 Jan;31(1):133-49. doi: 10.1093/humrep/dev279. Epub 2015 Nov 17. Hum Reprod. 2016. PMID: 26577303 Free PMC article.
-
Pre- and postovulatory aging of murine oocytes affect the transcript level and poly(A) tail length of maternal effect genes.PLoS One. 2014 Oct 1;9(10):e108907. doi: 10.1371/journal.pone.0108907. eCollection 2014. PLoS One. 2014. PMID: 25271735 Free PMC article.
-
In vitro postovulatory oocyte aging affects H3K9 trimethylation in two-cell embryos after IVF.Ann Anat. 2020 Jan;227:151424. doi: 10.1016/j.aanat.2019.151424. Epub 2019 Oct 11. Ann Anat. 2020. PMID: 31610252
-
Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization.Hum Reprod Update. 2015 Jul-Aug;21(4):427-54. doi: 10.1093/humupd/dmv011. Epub 2015 Mar 4. Hum Reprod Update. 2015. PMID: 25744083 Review.
-
Epigenetic changes associated with oocyte aging.Sci China Life Sci. 2012 Aug;55(8):670-6. doi: 10.1007/s11427-012-4354-3. Epub 2012 Aug 30. Sci China Life Sci. 2012. PMID: 22932882 Review.
Cited by
-
Oocyte quality following in vitro follicle development†.Biol Reprod. 2022 Feb 22;106(2):291-315. doi: 10.1093/biolre/ioab242. Biol Reprod. 2022. PMID: 34962509 Free PMC article. Review.
-
Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring.J Med Genet. 2018 Jul;55(7):497-504. doi: 10.1136/jmedgenet-2017-105190. Epub 2018 Mar 24. J Med Genet. 2018. PMID: 29574422 Free PMC article.
-
Expression of the histone lysine methyltransferases SETD1B, SETDB1, SETD2, and CFP1 exhibits significant changes in the oocytes and granulosa cells of aged mouse ovaries.Histochem Cell Biol. 2022 Jul;158(1):79-95. doi: 10.1007/s00418-022-02102-3. Epub 2022 Apr 20. Histochem Cell Biol. 2022. PMID: 35445296
-
The phenotypic variations of multi-locus imprinting disturbances associated with maternal-effect variants of NLRP5 range from overt imprinting disorder to apparently healthy phenotype.Clin Epigenetics. 2019 Dec 11;11(1):190. doi: 10.1186/s13148-019-0760-8. Clin Epigenetics. 2019. PMID: 31829238 Free PMC article.
-
Single-cell profiling of transcriptomic changes during in vitro maturation of human oocytes.Reprod Med Biol. 2022 May 9;21(1):e12464. doi: 10.1002/rmb2.12464. eCollection 2022 Jan-Dec. Reprod Med Biol. 2022. PMID: 35582522 Free PMC article.
References
-
- Smits LJ, Jongbloet PH, Zielhuis GA (1995) Preovulatory overripeness of the oocyte as a cause of ovarian dysfunction in the human female. Med Hypotheses 45: 441–448. - PubMed
-
- Cortvrindt RG, Smitz JE (2002) Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update 8: 243–254. - PubMed
-
- Adriaens I, Cortvrindt R, Smitz J (2004) Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod 19: 398–408. - PubMed
-
- Demant M, Trapphoff T, Frohlich T, Arnold GJ, Eichenlaub-Ritter U (2012) Vitrification at the pre-antral stage transiently alters inner mitochondrial membrane potential but proteome of in vitro grown and matured mouse oocytes appears unaffected. Hum Reprod 27: 1096–1111. 10.1093/humrep/der453 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

