Aldehyde dehydrogenases inhibition eradicates leukemia stem cells while sparing normal progenitors
- PMID: 27611922
- PMCID: PMC5056970
- DOI: 10.1038/bcj.2016.78
Aldehyde dehydrogenases inhibition eradicates leukemia stem cells while sparing normal progenitors
Abstract
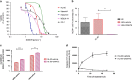
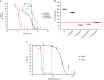
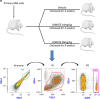
The vast majority of patients with acute myeloid leukemia (AML) achieve complete remission (CR) after standard induction chemotherapy. However, the majority subsequently relapse and die of the disease. A leukemia stem cell (LSC) paradigm has been invoked to explain this failure of CR to reliably translate into cure. Indeed, LSCs are highly enriched in CD34+CD38- leukemic cells that exhibit positive aldehyde dehydrogenase activity (ALDH+) on flow cytometry, these LSCs are resistant to currently existing treatments in AML such as cytarabine and anthracycline that, at the cost of great toxicity on normal cells, are highly active against the leukemic bulk, but spare the LSCs responsible for relapse. To try to combat the LSC population selectively, a well-characterized ALDH inhibitor by the trivial name of dimethyl ampal thiolester (DIMATE) was assessed on sorted CD34+CD38- subpopulations from AML patients and healthy patients. ALDH activity and cell viability were monitored by flow cytometry. From enzyme kinetic studies DIMATE is an active enzyme-dependent, competitive, irreversible inhibitor of ALDH1. On cells in culture, DIMATE is a powerful inhibitor of ALDHs 1 and 3, has a major cytotoxic activity on human AML cell lines. Moreover, DIMATE is highly active against leukemic populations enriched in LSCs, but, unlike conventional chemotherapy, DIMATE is not toxic for healthy hematopoietic stem cells which retained, after treatment, their self-renewing and multi-lineage differentiation capacity in immunodeficient mice, xenografted with human leukemic cells. DIMATE eradicates specifically human AML cells and spares healthy mouse hematologic cells.
Figures

Similar articles
-
The rarity of ALDH(+) cells is the key to separation of normal versus leukemia stem cells by ALDH activity in AML patients.Int J Cancer. 2015 Aug 1;137(3):525-36. doi: 10.1002/ijc.29410. Epub 2015 Jan 14. Int J Cancer. 2015. PMID: 25545165 Free PMC article.
-
Update of ALDH as a Potential Biomarker and Therapeutic Target for AML.Biomed Res Int. 2018 Jan 3;2018:9192104. doi: 10.1155/2018/9192104. eCollection 2018. Biomed Res Int. 2018. PMID: 29516013 Free PMC article. Review.
-
Variable aldehyde dehydrogenase activity and effects on chemosensitivity of primitive human leukemic cells.Exp Hematol. 2017 Mar;47:54-63. doi: 10.1016/j.exphem.2016.10.012. Epub 2016 Nov 5. Exp Hematol. 2017. PMID: 27826122
-
A clinically relevant population of leukemic CD34(+)CD38(-) cells in acute myeloid leukemia.Blood. 2012 Apr 12;119(15):3571-7. doi: 10.1182/blood-2011-06-364182. Epub 2012 Jan 19. Blood. 2012. PMID: 22262762 Free PMC article.
-
Acute Myeloid Leukemia Stem Cell Heterogeneity and Its Clinical Relevance.Adv Exp Med Biol. 2019;1139:153-169. doi: 10.1007/978-3-030-14366-4_9. Adv Exp Med Biol. 2019. PMID: 31134500 Review.
Cited by
-
Identification of Novel Isatin Derivative Bearing a Nitrofuran Moiety as Potent Multi-Isoform Aldehyde Dehydrogenase Inhibitor.Molecules. 2024 Jun 29;29(13):3114. doi: 10.3390/molecules29133114. Molecules. 2024. PMID: 38999066 Free PMC article.
-
Genome-Wide CRISPR Screening Identifies the Tumor Suppressor Candidate OVCA2 As a Determinant of Tolerance to Acetaldehyde.Toxicol Sci. 2019 May 1;169(1):235-245. doi: 10.1093/toxsci/kfz037. Toxicol Sci. 2019. PMID: 31059574 Free PMC article.
-
A Pan-ALDH1A Inhibitor Induces Necroptosis in Ovarian Cancer Stem-like Cells.Cell Rep. 2019 Mar 12;26(11):3061-3075.e6. doi: 10.1016/j.celrep.2019.02.032. Cell Rep. 2019. PMID: 30865894 Free PMC article.
-
Pilot Study of an Integrative New Tool for Studying Clinical Outcome Discrimination in Acute Leukemia.Front Oncol. 2019 Apr 9;9:245. doi: 10.3389/fonc.2019.00245. eCollection 2019. Front Oncol. 2019. PMID: 31024847 Free PMC article.
-
Anticancer Activity of Ω-6 Fatty Acids through Increased 4-HNE in Breast Cancer Cells.Cancers (Basel). 2021 Dec 20;13(24):6377. doi: 10.3390/cancers13246377. Cancers (Basel). 2021. PMID: 34944997 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. - PubMed
-
- Jemal A, Clegg LX, Ward E, Ries LAG, Wu X, Jamison PM et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer 2004; 101: 3–27. - PubMed
-
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474. - PubMed
-
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

