Androgen receptor differentially regulates the proliferation of prostatic epithelial cells in vitro and in vivo
- PMID: 27611945
- PMCID: PMC5342561
- DOI: 10.18632/oncotarget.11879
Androgen receptor differentially regulates the proliferation of prostatic epithelial cells in vitro and in vivo
Abstract
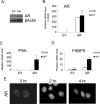
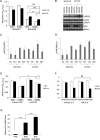
Androgens regulate the proliferation and differentiation of prostatic epithelial cells, including prostate cancer (PCa) cells in a context-dependent manner. Androgens and androgen receptor (AR) do not invariably promote cell proliferation; in the normal adult, endogenous stromal and epithelial AR activation maintains differentiation and inhibits organ growth. In the current study, we report that activation of AR differentially regulates the proliferation of human prostate epithelial progenitor cells, NHPrE1, in vitro and in vivo. Inducing AR signaling in NHPrE1 cells suppressed cell proliferation in vitro, concomitant with a reduction in MYC expression. However, ectopic expression of AR in vivo stimulated cell proliferation and induced development of invasive PCa in tissue recombinants consisting of NHPrE1/AR cells and rat urogenital mesenchymal (UGM) cells, engrafted under renal capsule of adult male athymic mice. Expression of MYC increased in the NHPrE1/AR recombinant tissues, in contrast to the reduction seen in vitro. The inhibitory effect of AR signaling on cell proliferation in vitro were reduced by co-culturing NHPrE1/AR epithelial cells with prostatic stromal cells. In conclusion, these studies revealed that AR signaling differentially regulates proliferation of human prostatic epithelia cells in vitro and in vivo through mechanisms involving stromal/epithelial interactions.
Keywords: androgen receptor; prostate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. - PubMed
-
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation; research in biological diversity. 2001;68:270–9. - PubMed
-
- Shabsigh A, Chang DT, Heitjan DF, Kiss A, Olsson CA, Puchner PJ, Buttyan R. Rapid reduction in blood flow to the rat ventral prostate gland after castration: preliminary evidence that androgens influence prostate size by regulating blood flow to the prostate gland and prostatic endothelial cell survival. Prostate. 1998;36:201–6. - PubMed
-
- Shabsigh A, Tanji N, D'Agati V, Burchardt T, Burchardt M, Hayek O, Shabsigh R, Buttyan R. Vascular anatomy of the rat ventral prostate. Anat Rec. 1999;256:403–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

