Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow-up
- PMID: 27611947
- PMCID: PMC5341887
- DOI: 10.18632/oncotarget.11883
Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow-up
Abstract
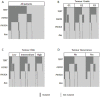
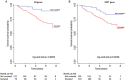
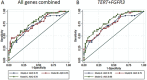
Most bladder cancer (BC) patients need life-long, invasive and expensive monitoring and treatment, making it a serious burden on the health system. Thus, there is a pressing need for an accurate test to assist diagnosis and surveillance of BC as an alternative to cystoscopy. Mutations in human TERT, FGFR3, PIK3CA, and RAS genes have been proposed as potential molecular markers in bladder tumor. Their concomitant presence in urine samples has not been fully explored.We investigated a panel of mutations in DNA from exfoliated urinary cells of 255 BC patients at diagnosis. Forty-one mutations in TERT, FGFR3, PIK3CA, and RAS were analyzed by SNaPshot assay in relation to clinical outcome. In 81 of these patients under surveillance, the same set of mutations was screened in additional 324 samples prospectively collected.The most common mutations detected in urine at diagnosis were in the TERT promoter. In non-invasive BC, these mutations were related to high risk and grade (p<0.0001) as well as progression to muscle-invasive disease (p=0.01), whereas FGFR3 mutations were observed in low-grade BC (p=0.02) and patients with recurrences (p=0.05). Stronger associations were observed for combined TERT and FGFR3 mutations and number of recurrences (OR: 4.54 95% CI: 1.23-16.79, p=0.02). Analyses of the area under the curve for combinations of mutations detected at diagnosis and follow-up showed an accuracy of prediction of recurrence of 0.80 (95% CI: 0.71-0.89).Mutations in urine of BC patients may represent reliable biomarkers. In particular, TERT and FGFR3 mutations have a good accuracy of recurrence prediction.
Keywords: TERT; bladder cancer; recurrence; urine mutation analyses.
Conflict of interest statement
No conflicts of interest were disclosed.
Figures
Similar articles
-
Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer.BMC Cancer. 2016 Sep 1;16(1):704. doi: 10.1186/s12885-016-2748-5. BMC Cancer. 2016. PMID: 27586786 Free PMC article.
-
The Diagnostic and Prognostic Performance of Urinary FGFR3 Mutation Analysis in Bladder Cancer Surveillance: A Prospective Multicenter Study.Urology. 2015 Dec;86(6):1185-90. doi: 10.1016/j.urology.2015.07.036. Epub 2015 Sep 11. Urology. 2015. PMID: 26364695
-
Increased accuracy of a novel mRNA-based urine test for bladder cancer surveillance.BJU Int. 2018 Jan;121(1):29-37. doi: 10.1111/bju.14019. Epub 2017 Oct 12. BJU Int. 2018. PMID: 28941000
-
Urinary markers in the everyday diagnosis of bladder cancer.Urologia. 2013 Sep-Dec;80(4):265-75. doi: 10.5301/urologia.5000041. Epub 2013 Nov 29. Urologia. 2013. PMID: 24419920 Review.
-
Replacing cystoscopy by urine markers in the follow-up of patients with low-risk non-muscle-invasive bladder cancer?-An International Bladder Cancer Network project.Urol Oncol. 2016 Oct;34(10):452-9. doi: 10.1016/j.urolonc.2016.06.001. Epub 2016 Jul 2. Urol Oncol. 2016. PMID: 27381893 Review.
Cited by
-
Validation of a Novel, Sensitive, and Specific Urine-Based Test for Recurrence Surveillance of Patients With Non-Muscle-Invasive Bladder Cancer in a Comprehensive Multicenter Study.Front Genet. 2019 Dec 18;10:1237. doi: 10.3389/fgene.2019.01237. eCollection 2019. Front Genet. 2019. PMID: 31921291 Free PMC article.
-
Diagnostic and Prognostic Potential of Biomarkers CYFRA 21.1, ERCC1, p53, FGFR3 and TATI in Bladder Cancers.Int J Mol Sci. 2020 May 9;21(9):3360. doi: 10.3390/ijms21093360. Int J Mol Sci. 2020. PMID: 32397531 Free PMC article. Review.
-
Photodynamic Diagnosis and Therapy in Non-Muscle-Invasive Bladder Cancer.Cancers (Basel). 2024 Jun 22;16(13):2299. doi: 10.3390/cancers16132299. Cancers (Basel). 2024. PMID: 39001362 Free PMC article. Review.
-
Phenotypic Analysis of Urothelial Exfoliated Cells in Bladder Cancer via Microfluidic Immunoassays: Sialyl-Tn as a Novel Biomarker in Liquid Biopsies.Front Oncol. 2020 Sep 16;10:1774. doi: 10.3389/fonc.2020.01774. eCollection 2020. Front Oncol. 2020. PMID: 33042825 Free PMC article.
-
Single-cell sequencing technologies in bladder cancer research: Applications and challenges.Front Genet. 2022 Oct 19;13:1027909. doi: 10.3389/fgene.2022.1027909. eCollection 2022. Front Genet. 2022. PMID: 36338973 Free PMC article. Review.
References
-
- Guillaume L, Guy L. [Epidemiology of and risk factors for bladder cancer and for urothelial tumors]. [Article in French] Rev Prat. 2014;64:1372–1374. 1378-1380. - PubMed
-
- Eble J.N. SG, Epstein J.I., Sesterhenn I.A., editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; 2004. World Health Organization Classification of Tumours.
-
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. - PubMed
-
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. discussion 475-467. - PubMed
-
- Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, van Rhijn BW, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2016 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

