F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis
- PMID: 27611949
- PMCID: PMC5325436
- DOI: 10.18632/oncotarget.11887
F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis
Abstract
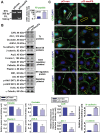
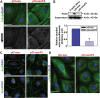
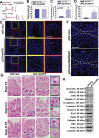
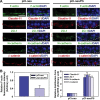
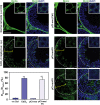
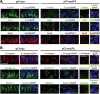
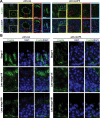
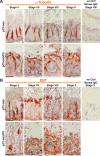
During the release of sperm at spermiation, a biologically active F5-peptide, which can disrupt the Sertoli cell tight junction (TJ) permeability barrier, is produced at the site of the degenerating apical ES (ectoplasmic specialization). This peptide coordinates the events of spermiation and blood-testis barrier (BTB) remodeling at stage VIII of the epithelial cycle, creating a local apical ES-BTB axis to coordinate cellular events across the epithelium. The mechanism(s) by which F5-peptide perturbs BTB restructuring, and its involvement in apical ES dynamics remain unknown. F5-peptide, besides perturbing BTB integrity, was shown to induce germ cell release from the epithelium following its efficient in vivo overexpression in the testis. Overexpression of F5-peptide caused disorganization of actin- and microtubule (MT)-based cytoskeletons, mediated by altering the spatiotemporal expression of actin binding/regulatory proteins in the seminiferous epithelium. F5-peptide perturbed the ability of actin microfilaments and/or MTs from converting between their bundled and unbundled/defragmented configuration, thereby perturbing adhesion between spermatids and Sertoli cells. Since apical ES and basal ES/BTB are interconnected through the underlying cytoskeletal networks, this thus provides an efficient and novel mechanism to coordinate different cellular events across the epithelium during spermatogenesis through changes in the organization of actin microfilaments and MTs. These findings also illustrate the potential of F5-peptide being a male contraceptive peptide for men.
Keywords: F5-peptide; actin cytoskeleton; ectoplasmic specialization; microtubule cytoskeleton; testis.
Conflict of interest statement
Nothing to declare.
Figures
Similar articles
-
Basement Membrane Laminin α2 Regulation of BTB Dynamics via Its Effects on F-Actin and Microtubule Cytoskeletons Is Mediated Through mTORC1 Signaling.Endocrinology. 2017 Apr 1;158(4):963-978. doi: 10.1210/en.2016-1630. Endocrinology. 2017. PMID: 28323988 Free PMC article.
-
Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis.FASEB J. 2015 Sep;29(9):3788-805. doi: 10.1096/fj.14-267997. Epub 2015 Jun 5. FASEB J. 2015. PMID: 26048141 Free PMC article.
-
F5-peptide enhances the efficacy of the non-hormonal male contraceptive adjudin.Contraception. 2019 Jun;99(6):350-356. doi: 10.1016/j.contraception.2019.01.007. Epub 2019 Feb 11. Contraception. 2019. PMID: 30763581 Free PMC article.
-
Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons.Tissue Barriers. 2016 Nov 28;4(4):e1265042. doi: 10.1080/21688370.2016.1265042. eCollection 2016. Tissue Barriers. 2016. PMID: 28123928 Free PMC article. Review.
-
NC1-peptide derived from collagen α3 (IV) chain is a blood-tissue barrier regulator: lesson from the testis.Asian J Androl. 2021 Mar-Apr;23(2):123-128. doi: 10.4103/aja.aja_44_20. Asian J Androl. 2021. PMID: 32896837 Free PMC article. Review.
Cited by
-
Autophagy regulation and protein kinase activity of PIK3C3 controls sertoli cell polarity through its negative regulation on SCIN (scinderin).Autophagy. 2023 Nov;19(11):2934-2957. doi: 10.1080/15548627.2023.2235195. Epub 2023 Jul 14. Autophagy. 2023. PMID: 37450577 Free PMC article.
-
mTORC1/rpS6 and spermatogenic function in the testis-insights from the adjudin model.Reprod Toxicol. 2019 Oct;89:54-66. doi: 10.1016/j.reprotox.2019.07.002. Epub 2019 Jul 3. Reprod Toxicol. 2019. PMID: 31278979 Free PMC article.
-
Vangl2 regulates spermatid planar cell polarity through microtubule (MT)-based cytoskeleton in the rat testis.Cell Death Dis. 2018 Mar 1;9(3):340. doi: 10.1038/s41419-018-0339-x. Cell Death Dis. 2018. PMID: 29497043 Free PMC article.
-
Basement Membrane Laminin α2 Regulation of BTB Dynamics via Its Effects on F-Actin and Microtubule Cytoskeletons Is Mediated Through mTORC1 Signaling.Endocrinology. 2017 Apr 1;158(4):963-978. doi: 10.1210/en.2016-1630. Endocrinology. 2017. PMID: 28323988 Free PMC article.
-
Regulation of spermatogenesis by a local functional axis in the testis: role of the basement membrane-derived noncollagenous 1 domain peptide.FASEB J. 2017 Aug;31(8):3587-3607. doi: 10.1096/fj.201700052R. Epub 2017 May 9. FASEB J. 2017. PMID: 28487282 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

