Patient and Partner Feedback Reports to Improve Statin Medication Adherence: A Randomized Control Trial
- PMID: 27612487
- PMCID: PMC5330995
- DOI: 10.1007/s11606-016-3858-0
Patient and Partner Feedback Reports to Improve Statin Medication Adherence: A Randomized Control Trial
Abstract
Background: Simple nudges such as reminders and feedback reports to either a patient or a partner may facilitate improved medication adherence.
Objective: To test the impact of a pill bottle used to monitor adherence, deliver a daily alarm, and generate weekly medication adherence feedback reports on statin adherence.
Design: Three-month, three-arm randomized clinical trial (ClinicalTrials.gov identifier: NCT02480530).
Participants: One hundred and twenty-six veterans with known coronary artery disease and poor adherence (medication possession ratio <80 %).
Intervention: Patients were randomized to one of three groups: (1) a control group (n = 36) that received a pill-monitoring device with no alarms or feedback; (2) an individual feedback group (n = 36) that received a daily alarm and a weekly medication adherence feedback report; and (3) a partner feedback group (n = 54) that received an alarm and a weekly feedback report that was shared with a friend, family member, or a peer. The intervention continued for 3 months, and participants were followed for an additional 3 months after the intervention period.
Main measures: Adherence as measured by pill bottle. Secondary outcomes included change in LDL (mg/dl), patient activation, and social support.
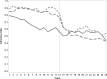
Key results: During the 3-month intervention period, medication adherence was higher in both feedback arms than in the control arm (individual feedback group 89 %, partner feedback group 86 %, control group 67 %; p < 0.001 and = 0.001). At 6 months, there was no difference in medication adherence between either of the feedback groups and the control (individual feedback 60 %, partner feedback 52 %, control group 54 %; p = 0.75 and 0.97).
Conclusions: Daily alarms combined with individual or partner feedback reports improved statin medication adherence. While neither an individual feedback nor partner feedback strategy created a sustainable medication adherence habit, the intervention itself is relatively easy to implement and low cost.
Keywords: medication adherence; social force; social support; statins.
Conflict of interest statement
Funders
This work was supported by a grant from the Center for the Evaluation of Patient Aligned Care Teams at the Philadelphia VA.
Conflicts of Interest
Kevin Volpp is a principal at the behavioral economics consulting firm VAL Health, and has received consulting income and research support from CVS Health, as well as research support from Humana, Weight Watchers, and the Vitality Institute (not related to Vitality, Inc., the manufacturer of the GlowCap pill bottle). Dr. Asch has also served as a consultant for VAL Health. All the other authors declare that they do not have a conflict of interest.
Figures
Comment in
-
Improving Medication Adherence: Keep Your Eyes on the Prize.J Gen Intern Med. 2017 Mar;32(3):236-237. doi: 10.1007/s11606-016-3927-4. J Gen Intern Med. 2017. PMID: 27848188 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

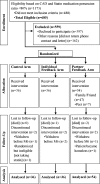
 Control
Control  Individual…
Individual…  Partner feedback.
Partner feedback. 