Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering
- PMID: 27612767
- PMCID: PMC5019123
- DOI: 10.1016/j.msec.2016.07.060
Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering
Abstract
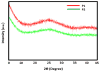
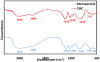
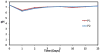
In this study we developed carboxymethyl cellulose (CMC) microparticles through ionic crosslinking with the aqueous ion complex of zirconium (Zr) and further complexing with chitosan (CS) and determined the physio-chemical and biological properties of these novel microparticles. In order to assess the role of Zr, microparticles were prepared in 5% and 10% (w/v) zirconium tetrachloride solution. Scanning electron microscopy (SEM) with energy dispersive X-ray spectrometer (EDS) results showed that Zr was uniformly distributed on the surface of the microparticles as a result of which uniform groovy surface was obtained. We found that Zr enhances the surface roughness of the microparticles and stability studies showed that it also increases the stability of microparticles in phosphate buffered saline. The crosslinking of anionic CMC with cationic Zr and CS was confirmed by Fourier transform infrared spectroscopy (FTIR) results. The response of murine pre-osteoblasts (OB-6) when cultured with microparticles was investigated. Live/dead cell assay showed that microparticles did not induce any cytotoxic effects as cells were attaching and proliferating on the well plate as well as along the surface of microparticles. In addition, SEM images showed that microparticles support the attachment of cells and they appeared to be directly interacting with the surface of microparticle. Within 10days of culture most of the top surface of microparticles was covered with a layer of cells indicating that they were proliferating well throughout the surface of microparticles. We observed that Zr enhances the cell attachment and proliferation as more cells were present on microparticles with 10% Zr. These promising results show the potential applications of CMC-Zr microparticles in bone tissue engineering.
Keywords: Carboxymethyl cellulose; Microparticles; Pre-osteoblasts; Surface roughness; Zirconium.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures










References
-
- Sannino A, Demitri C, Madaghiele M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials. 2009:353–73.
-
- Pasqui D, Torricelli P, De Cagna M, Fini M, Barbucci R. Carboxymethyl cellulose-hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J Biomed Mater Res A. 2014;102:1568–79. - PubMed
-
- Raucci MG, Alvarez-Perez MA, Demitri C, Giugliano D, De Benedictis V, Sannino A, et al. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. Journal of Biomedical Materials Research Part A. 2015;103:2045–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

