International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials
- PMID: 27613807
- PMCID: PMC5344772
- DOI: 10.1136/annrheumdis-2016-210242
International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials
Abstract
Objective: To identify a core set of domains (outcomes) to be measured in psoriatic arthritis (PsA) clinical trials that represent both patients' and physicians' priorities.
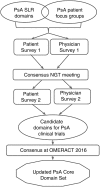
Methods: We conducted (1) a systematic literature review (SLR) of domains assessed in PsA; (2) international focus groups to identify domains important to people with PsA; (3) two international surveys with patients and physicians to prioritise domains; (4) an international face-to-face meeting with patients and physicians using the nominal group technique method to agree on the most important domains; and (5) presentation and votes at the Outcome Measures in Rheumatology (OMERACT) conference in May 2016. All phases were performed in collaboration with patient research partners.
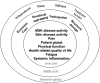
Results: We identified 39 unique domains through the SLR (24 domains) and international focus groups (34 domains). 50 patients and 75 physicians rated domain importance. During the March 2016 consensus meeting, 12 patients and 12 physicians agreed on 10 candidate domains. Then, 49 patients and 71 physicians rated these domains' importance. Five were important to >70% of both groups: musculoskeletal disease activity, skin disease activity, structural damage, pain and physical function. Fatigue and participation were important to >70% of patients. Patient global and systemic inflammation were important to >70% of physicians. The updated PsA core domain set endorsed by 90% of OMERACT 2016 participants includes musculoskeletal disease activity, skin disease activity, pain, patient global, physical function, health-related quality of life, fatigue and systemic inflammation.
Conclusions: The updated PsA core domain set incorporates patients' and physicians' priorities and evolving PsA research. Next steps include identifying outcome measures that adequately assess these domains.
Keywords: Outcomes research; Psoriatic Arthritis; Qualitative research; Spondyloarthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures

References
-
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34:1167–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

