Targeting Liver Fibrosis with a Cell-penetrating Protease-activated Receptor-2 (PAR2) Pepducin
- PMID: 27613872
- PMCID: PMC5087736
- DOI: 10.1074/jbc.M116.732743
Targeting Liver Fibrosis with a Cell-penetrating Protease-activated Receptor-2 (PAR2) Pepducin
Abstract
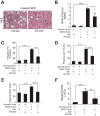
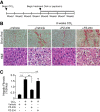
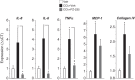
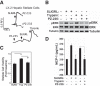
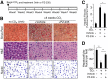
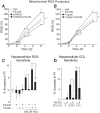
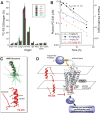
Chronic liver inflammation and fibrosis in nonalcoholic steatohepatitis can lead to cirrhosis and liver failure for which there are currently no approved treatments. Protease-activated receptor-2 (PAR2) is an emerging new target expressed on liver stellate cells and hepatocytes that regulates the response to liver injury and inflammation. Here, we identified a pepducin to block the deleterious actions of PAR2 in promoting liver fibrosis. Non-alcoholic fatty liver disease and early fibrosis were induced by the methionine-choline-deficient diet in mice. Fibrotic liver disease was induced by administering carbon tetrachloride for 8 weeks. Mice were treated with the pepducin PZ-235 either from onset of the experiment or after fibrosis was established. Hepatic fibrosis, collagen content, inflammatory cytokines, steatosis, triglycerides, and NAFLD activity score were assessed as primary outcome parameters depending on the model. The activity of the PAR2 pepducin on cultured stellate cell activation and hepatocyte reactive oxygen species production was evaluated. PZ-235 significantly suppressed liver fibrosis, collagen deposition, inflammatory cytokines, NAFLD activity score, steatosis, triglycerides, aspartate transaminase, alanine transaminase, and stellate cell proliferation by up to 50-100%. The PAR2 inhibitor afforded significant protective effects against hepatocellular necrosis and attenuated PAR2-mediated reactive oxygen species production in hepatocytes. PZ-235 was distributed to liver and other mouse tissues and was found to form a well structured α-helix that closely resembles the juxtamembrane helical region of the analogous TM6 and third intracellular region of the intact receptor that is critical for coupling to internal G proteins. The ability of PZ-235 to effectively suppress fibrosis, hepatocellular necrosis, reactive oxygen species production, steatosis, and inflammation indicates the potential for PAR2 pepducin inhibitors to be broadly efficacious in the treatment of liver fibrosis.
Keywords: G protein-coupled receptor (GPCR); PAR2; biotechnology; cell-penetrating peptide (CPP); fibrosis; pepducin; reactive oxygen species (ROS).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- LaBrecque D., Abbas A., Anania F., Ferenci P., Ghafoor-Khan A., Goh K.-L., Hamid S. S., Isakov V., Lizarzabal M., Mojica-Pernaranda M., Rivera-Ramos J. F., Sarin S., Stimac D., Thomson A. B., Umar M., et al. (2012) Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, in World Gastroenterology Organisation Global Guidelines (World Gastroenterology Organisation, ed) World Gastroenterology Organisation, Milwaukee, WI - PubMed
-
- Fujii H., and Kawada N. (2012) Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 47, 215–225 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

