A Randomized Trial of Adjunct mHealth Abstinence Reinforcement With Transdermal Nicotine and Counseling for Smoking Cessation
- PMID: 27613901
- PMCID: PMC6075519
- DOI: 10.1093/ntr/ntw155
A Randomized Trial of Adjunct mHealth Abstinence Reinforcement With Transdermal Nicotine and Counseling for Smoking Cessation
Abstract
Introduction: Abstinence reinforcement is efficacious for improving smoking treatment outcomes, but practical constraints related to the need for multiple in-person carbon monoxide (CO) breath tests daily to verify smoking abstinence have limited its use. This study tested an mHealth procedure to remotely monitor and reinforce smoking abstinence in individuals' natural environment.
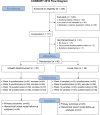
Methods: Eligible treatment-seeking smokers (N = 90) were randomized to (1) usual care and ecological monitoring with abstinence reinforcement (mHealth reinforcement) or (2) without reinforcement (mHealth monitoring). Usual care was 8 weeks of transdermal nicotine and twice-weekly telephone counseling. Following training, an interactive voice response system prompted participants to conduct CO tests 1-3 daily at pseudorandom times (7 am to 10 pm) for 4 weeks. When prompted, participants used a study cell phone and CO monitor to complete a CO self-test, video record the process, and submit videos using multimedia messaging. mHealth reinforcement participants could earn prizes for smoking-negative on-time CO tests. The interactive voice response generated preliminary earnings immediately. Earnings were finalized by comparing video records against participants' self-reports.
Results: mHealth reinforcement was associated with a greater proportion of smoking-negative CO tests, longest duration of prolonged abstinence, and point-prevalence abstinence during the monitoring/reinforcement phase compared to mHealth monitoring (p < .01, d = 0.8-1.3). Follow-up (weeks 4-24) analyses indicated main effects of reinforcement on point-prevalence abstinence and proportion of days smoked (p ≤ .05); values were comparable by week 24.
Conclusions: mHealth reinforcement has short-term efficacy. Research on methods to enhance and sustain benefits is needed.
Implications: This study suggests that mHealth abstinence reinforcement is efficacious and may present temporal and spatial opportunities to research, engage, and support smokers trying to quit that do not exist with conventional (not technology-based) reinforcement interventions.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58(1–2):9–25. doi:10.1016/S0376-8716(99)00071-X. - PubMed
-
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi:10.1111/j.1360-0443.2006.01311.x. - PubMed
-
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi:10.1111/j.1360-0443.2006.01581.x. - PubMed
-
- Dutra L, Dutra L, Stathopoulou G, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi:10.1176/appi.ajp.2007.06111851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical