Present Yourself! By MHC Class I and MHC Class II Molecules
- PMID: 27614798
- PMCID: PMC5159193
- DOI: 10.1016/j.it.2016.08.010
Present Yourself! By MHC Class I and MHC Class II Molecules
Abstract
Since the discovery of MHC molecules, it has taken 40 years to arrive at a coherent picture of how MHC class I and MHC class II molecules really work. This is a story of the proteases and MHC-like chaperones that support the MHC class I and II molecules in presenting peptides to the immune system. We now understand that the MHC system shapes both the repertoire of presented peptides and the subsequent T cell response, with important implications ranging from transplant rejection to tumor immunotherapies. Here we present an illustrated review of the ins and outs of MHC class I and MHC class II antigen presentation.
Keywords: MHC class I; MHC class II; antigen presentation; autoimmune diseases; transplantation; tumor immunology.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


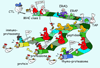

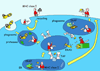
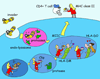
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

