Insulin Signalling: The Inside Story
- PMID: 27614806
- PMCID: PMC5272803
- DOI: 10.1016/j.jcjd.2016.07.002
Insulin Signalling: The Inside Story
Abstract
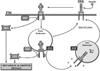
Insulin signalling begins with binding to its cell surface insulin receptor (IR), which is a tyrosine kinase. The insulin receptor kinase (IRK) is subsequently autophosphorylated and activated to tyrosine phosphorylate key cellular substrates that are essential for entraining the insulin response. Although IRK activation begins at the cell surface, it is maintained and augmented following internalization into the endosomal system (ENS). The peroxovanadium compounds (pVs) were discovered to activate the IRK in the absence of insulin and lead to a full insulin response. Thus, IRK activation is both necessary and sufficient for insulin signalling. Furthermore, this could be shown to occur with activation of only the endosomal IRK. The mechanism of pV action was shown to be the inhibition of IRK-associated phosphotyrosine phosphatases (PTPs). Our studies showed that the duration and intensity of insulin signalling are modulated within ENS by the recruitment of cellular substrates to ENS; intra-endosomal acidification, which promotes dissociation of insulin from the IRK; an endosomal acidic insulinase, which degrades intra-endosomal insulin; and IRK-associated PTPs, which dephosphorylate and, hence, deactivate the IRK. Therefore, the internalization of IRKs is central to insulin signalling and its regulation.
La signalisation de l’insuline commence par la liaison de l’insuline à son récepteur (IR) situé à la surface des cellules, soit la tyrosine kinase. La kinase du récepteur de l’insuline (KRI) s’est subséquemment autophosphorylée et activée vers les principaux substrats cellulaires de la tyrosine phosphorylée qui sont essentiels au déclenchement de la réponse à l’insuline. Bien que l’activation de la KRI commence à la surface des cellules, elle est maintenue et augmentée à la suite de l’internalisation du système endosomal (SEN). Il a été découvert que les composés de peroxovanadium (pV) activent la KRI en l’absence d’insuline et mènent à une réponse insulinique complète. Par conséquent, l’activation de la KRI est nécessaire et suffisante à la signalisation de l’insuline. De plus, il pourrait être démontré qu’elle apparaît avec l’activation de la KRI endosomale seulement. Il a été démontré que le mécanisme de l’activité du pV est l’inhibition des phosphotyrosines phosphatases (PTP) associées à la KRI. Nos études ont montré que la durée et l’intensité de la signalisation de l’insuline sont modulées au sein du SEN par le recrutement de substrats cellulaires du SEN; l’acidification intra-endosomale, qui favorise la dissociation de l’insuline de la KRI; une insulinase endosomique acide, qui dégrade l’insuline dans les endosomes; les PTP associées à la KRI, qui déphosphorylent et, donc, désactivent la KRI. Par conséquent, l’internalisation des KRI est essentielle au déclenchement de la signalisation de l’insuline et à sa régulation.
Keywords: endosomal acidic insulinase; endosomes; insulin receptor; insulinase endosomique acide; phosphorylation; phosphotyrosine phosphatase; récepteur de l'insuline; tyrosine kinase.
Copyright © 2016 Canadian Diabetes Association. Published by Elsevier Inc. All rights reserved.
Figures
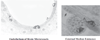

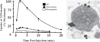



References
-
- Posner BI, Kelly PA, Shiu RP, Friesen HG. Studies of insulin, growth hormone and prolactin binding: Tissue distribution, species variation and characterization. Endocrinology. 1974;95:521–531. - PubMed
-
- Van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature. 1979;282:623–625. - PubMed
-
- Van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science. 1980;207:1081–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

