Synthesis and biological evaluation of santacruzamate A analogues for anti-proliferative and immunomodulatory activity
- PMID: 27614919
- PMCID: PMC5065774
- DOI: 10.1016/j.bmc.2016.08.040
Synthesis and biological evaluation of santacruzamate A analogues for anti-proliferative and immunomodulatory activity
Abstract
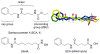
Santacruzamate A (SCA) is a natural product isolated from a Panamanian marine cyanobacterium, previously reported to have potent and selective histone deacetylase (HDAC) activity. To optimize the enzymatic and cellular activity, 40 SCA analogues were synthesized in a systematic exploration of the zinc-binding group (ZBG), cap terminus, and linker region. Two cap group analogues inhibited proliferation of MCF-7 breast cancer cells, with analogous increased degranulation of cytotoxic T cells (CTLs), while one cap group analogue reduced CTL degranulation, indicative of suppression of the immune response. Additional testing of these analogues resulted in reevaluation of the previously reported SCA mechanism of action. These analogues and the resulting structure-activity relationships will be of interest for future studies on cell proliferation and immune modulation.
Keywords: Anti-proliferative activity; Enzyme assays; Immune modulation; Natural product analogues; Santacruzamate A.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures






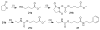


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

